Abstract
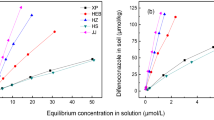
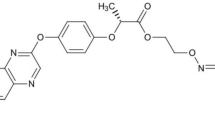
The effect of coexisting pesticide on adsorption/desorption and mobility of another one was investigated with carbendazim (CBD), imidacloprid (IDP), and atrazine (ATR). The data indicated that adsorption of CBD, ATR, and IDP on the tested soil was fitted well by Freundlich equation and increased with an order of IDP < ATR ≪ CBD. Adsorption of a pesticide was decreased by the coexistence of another one through their competitive adsorption. The presence of coexisting solute of the more adsorbability played a more important role than that of the lesser adsorbability. The adsorption of IDP and ATR was easier to be affected by 28.9–52.0 % and 31.1–60.7 % with the addition of CBD, while that of CBD was much less influenced by 3.4–18.1 % and 6.9–31.8 % with the presence of ATR and IDP, respectively. An adsorbability-related enhancement in desorption of the three pesticides by the co-adsorbed solute was also observed. As a result of competitive adsorption/desorption, the mobility of the pesticides estimated from soil thin-layer chromatography was altered. The results clearly illustrated that adsorbability and concentration-related alteration in adsorption/desorption and mobility will be caused by the coexistence of pesticides.











Similar content being viewed by others
References
Abate G, Masini JC (2005) Adsorption of atrazine, hydroxyatrazine, deethylatrazine, and deisopropylatrazine onto Fe(III) polyhydroxy cations intercalated Vermiculite and Montmorillonite. J Agric Food Chem 53(5):1612–1619
Boivin A, Cherrier R, Schiavon M (2005) A comparison of five pesticides adsorption and desorption processes in thirteen contrasting field soils. Chemosphere 61(5):668–676
Budavari S (1989) The Merck Index, 11th edn. Merck and CO, New Jersey
Chen JP, Wu SN, Chong KH (2003) Surface modification of a granular activated carbon by citric acid for enhancement of copper adsorption. Carbon 41:1979–1986
Chen JP, Pehkonen SO, Lau CC (2004) Phorate and terbufos adsorption onto four tropical soils. Colloid and Surfaces A 240:55–61
Chiou CT, Peters LJ, Freed VH (1979) A physical concept of soil–water equilibria for nonionic organic compounds. Science 206(4430):831–832
Davidse LC (1986) Benzimidazole fungicides: mechanism of action and biological impact. Annu Rev Phytopathol 24:43–65
Ellis SR, Hodson ME, Wege P (2007) The influence of different artificial soil types on the acute toxicity of carbendazim to the earthworm Eisenia fetida in laboratory toxicity tests. Eur J Soil Biol 43(1):239–245
Helling CS, Turner BC (1968) Pesticide mobility: determination by soil thin layer chromatography. Science 162(3853):562–563
Jeong CY, Selim HM (2011) Adsorption-desorption kinetics of imidacloprid in soils: influence of phosphate. Soil Sci 176:582–588
Kovaios ID, Paraskeva CA, Koutsoukos PG (2011) Adsorption of atrazine from aqueous electrolyte solutions on humic acid and silica. J Colloid Interf Sci 356:277–285
Leistra M, Matser AM (2005) Adsorption, transformation, and bioavailability of the fungicides carbendazim and iprodione in soil, alone and in combination. J Environ Sci Heal B 39(1):1–17
Li XH, Zhou QX, Wei SH, Ren WJ, Sun XY (2011) Adsorption and desorption of carbendazim and cadmium in typical soils in northeastern China as affected by temperature. Geoderma 160:347–354
Liu GS (1996) Physical and chemical analysis of soils and profile description. China Standard Publishing House, Beijing
Liu WP, Zheng W, Gan JY (2002) Competitive sorption between imidacloprid and imidacloprid-urea on soil clay minerals and humic acids. J Agr Food Chem 50(23):6823–6827
Lynch MR (1995) Procedures for assessing the environmental fate and ecotoxicity of pesticides. Society of Environmental Toxicology and Chemistry, SETAC Europe, Brussels, pp 9–10, 23–24
Müller K, Magesan BNS (2007) A critical review of the influence of effluent irrigation on the fate of pesticides in soil. Agr Ecosyst Environ 120:93–116
Nemeth-Konda L, Fuleky G, Morovjan G, Csokan P (2002) Sorption behaviour of acetochlor, atrazine, carbendazim, diazinon, imidacloprid and isoproturon on Hungarian agricultural soil. Chemosphere 48(5):545–552
OECD (2000) OECD guideline for the testing of chemicals: adsorption–desorption using a batch equilibrium method. OECD, Paris
Pateiro-Mourea M, Arias-Estéveza M, Simal-Gándarab J (2010) Competitive and non-competitive adsorption/desorption of paraquat, diquat and difenzoquat in vineyard-devoted soils. J Hazard Mater 178:194–201
Pignatello JJ (1991) Competitive effects in the sorption of nonpolar organic compound by soils. In: Baker RA (ed) Organic Substances and Sediments in Water. Lewis Publishers, Chelsea, pp 291–307
Ravanel P, Liegeois MH, Chevallier D, Tissut M (1999) Soil thin-layer chromatography and pesticide mobility through soil microstructures: new technical approach. J Chromatogr A 864:145–54
Rotich HK, Zhang Z, Zhao Y, Li J (2004) The adsorption behavior of three organophosphorus pesticides in peat and soil samples and their degradation in aqueous solutions at different temperatures and pH values. Int J Environ An Ch 84(4):289–301
Rütters H, Höllrigl-Rosta A, Kreuzig R, Bahadir M (1999) Sorption behavior of prochloraz in different soils. J Agric Food Chem 47:1242–1246
Tiktak A (2000) Application of pesticide leaching models to the Vredepeel dataset: II pesticide fate. Agr Water Manage 44:119–134
Tomlin C (1994) A world compendium the pesticide manual incorporating the agrochemical handbook. The Pesticide Manual British Crop Protection Council, Surrey, UK, 10th. pp 451–452
Xing BS, Pignatello JJ, Gigliotti B (1996) Competitive sorption between atrazine and other organic compounds in soils and model sorbents. Environ Sci Technol 30(8):2432–2440
Yu XY, Ying GG, Kookana RS (2006) Sorption and desorption behaviors of diuron in soils amended with charcoal. J Agr Food Chem 54(22):8545–8550
Acknowledgments
The present study was supported by the Special Fund for Environmental Scientific Research in the Public Interest (201109018), the National Natural Science Foundation of China (21177111, 20877068), the Major State Basic Research Development Program of China (2009CB119006), and National Key Technology R & D Program of China (NC2010BF0006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Jin, X., Ren, J., Wang, B. et al. Impact of coexistence of carbendazim, atrazine, and imidacloprid on their adsorption, desorption, and mobility in soil. Environ Sci Pollut Res 20, 6282–6289 (2013). https://doi.org/10.1007/s11356-013-1657-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1657-2