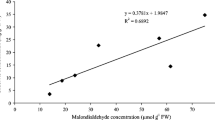
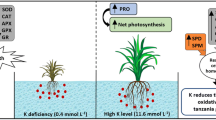
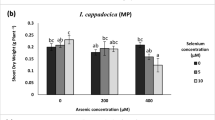
Abstract
In a hydroponic culture, experiments were performed to study the influence of potassium (K) supplementation (0, 20, 40, 60, 80, and 100 mg L−1) on the arsenic (As; 0, 8, and 10 mg L−1)-accrued changes in growth traits (plant biomass, root–shoot length) and the contents of lepidine, As and K, in garden cress (Lepidium sativum Linn.) at 10 days after treatment. The changes in these traits were correlated with shoot proline content, protein profile, and the activities of antioxidant enzymes namely superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), glutathione reductase (GR, EC 1.8.1.7), and ascorbate peroxidase (APX, EC 1.11.1.11). In general, As-alone treatments significantly decreased the growth traits but lead to significant enhancements in shoot proline and enzyme activities. K-supplementation to As-treated L. sativum seedlings decreased shoot-As content, reduced As-induced decreases in growth traits but enhanced the content of shoot proline, and the activities of the studied enzymes maximally with K100 + As8 and As10 mg L−1. Both 8 and 10 mg L−1 of As drastically downregulated the shoot proteins ranging from 43–65 kDa. With As10 mg L−1, there was a total depletion of protein bands below 23 kDa; however, K80 mg L−1 maximally recovered and upregulated the protein bands. Additionally, protein bands were downregulated (at par with As-alone treatment) above K80 mg L−1 level. Interestingly, As-stress increased lepidine content in a dose-dependent manner which was further augmented with the K-supplementation. It is suggested that K protects L. sativum against As-toxicity by decreasing its accumulation and strengthening antioxidant defense system and protein stability.



Similar content being viewed by others
References
Abou El-Nour MS, Saeed MA, Morsy MA (2000) Effect of potassium fertilization under two planting dates on yield, yield components, and some technological and chemical properties of Giza 80 cotton cultivar. Egypt J Agric Res 78:1219–1231
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:122–126
Alia P, Saradhi P (1991) Proline accumulation under heavy metal stress. J Plant Physiol 138:554–558
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008) Sulfur protects mustard (Brasasica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul 54:271–279
Anjum NA, Umar S, Iqbal M, Khan NA (2011) Cadmium causes oxidative stress in moongbean [Vigna radiata (L.) Wilczek] by affecting antioxidant enzyme systems and ascorbate-glutathione cycle metabolism. Russ J Plant Physiol 58:92–99
Anjum NA, Ahmad I, Mohmood I, Pacheco M, Duarte AC, Pereira E, Umar S, Ahmad A, Khan NA, Iqbal M, Prasad MNV (2012) Modulation of glutathione and its related enzymes in plants' responses to toxic metals and metalloids - a review. Environ Exp Bot 75:307–324
Anjum NA, Ahmad I, Rodrigues SM, Henriques B, Cruz N, Coelho C, Pacheco M, Duarte AC, Pereira E (2013) Eriophorum angustifolium and Lolium perenne metabolic adaptations to metals- and metalloids-induced anomalies in the vicinity of a chemical industrial complex. Environ Sci Pollut Res 20:568–581
Baccouch S, Chaoui A, El Ferjani E (1998) Nickel toxicity: effects on growth and metabolism of maize. J Plant Nutr 21:577–588
Barceló J, Poschenrieder C (1990) Plant water relations as affected by heavy metal stress: a review. J Plant Nutr 13:1–37
Bednarz CW, Oosterhuis DM (1999) Physiological changes associated with potassium deficiency in cotton. J Plant Nutr 22:303–313
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein - dye binding. Anal Biochem 72:248–254
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Cao X, Ma LQ, Shiralipour A (2003) Effect of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator Pteris vittata L. Environ Pollut 126:157–67
Chou IT, Chen CT, Kao CH (1991) Characteristics of the induction of the accumulation of proline by abscisic acid and isobutyric acid in detached rice leaves. Plant Cell Physiol 32:269–272
Demeyer K (1997) Nitrogen and alkaloid accumulation and partitioning in Datura stramonium L. J Herbs Spices Med Plants 5:15–23
Dhindsa RH, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid per oxidation, and decreased level SOD and CAT. J Exp Bot 52:1101–1109
Flora S (1999) Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2, 3-dimercaptosuccinic acid in rats. Clin Exp Pharm Physiol 26:865–869
Foyer CH, Halliwell B (1976) The presence of glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gabrielli R, Pandolfini T, Vergano O, Palandri MR (1990) Comparison of two serpentine species with different nickel tolerance strategies. Plant Soil 122:671–693
Ghourab MHH, Wassel OMM, Raya NAA (2000) Response of cotton plants to foliar application of (Pottasin-P)TM under two levels of nitrogen fertilizer. Egypt J Agric Res 78:781–93
Gulz PA, Gupta SK, Schulin R (2005) Arsenic accumulation of common plants from contaminated soils. Plant Soil 272:337–347
Gunes A, Pilbeam DJ, Inal A (2009) Effect of arsenic-phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil 314:211–220
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:713–722
Jaleel CA, Gopi R, Alagulakshmanan GM, Panneerselvam R (2006) Triadimefon induced changes in the antioxidant metabolism and ajmalicine production in Catharanthus roseus (L.) G. Don. Plant Sci 171:271–276
Jaleel CA, Panneerselvam R (2007) Variations in the antioxidative and indole alkaloid status in different parts of two varieties of Catharanthus roseus, an important folk herb. Chinese J Pharmacol Toxicol 21:487–494
Jin JW, Xu YF, Huang YF (2010) Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. African J Biotech 9:1619–1627
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC Press, Boca Raton
Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K (2002) Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem 277:3829–3835
Krackhardt M, Guerrier G (1995) Effect of osmotic and ionic stresses on proline and organic acid contents during imbibition and germination of soybean seeds. J Plant Physiol 146:725–730
Kurkdjian A, Guern J (1989) Intracellular pH: Measurement and importance in cell activity. Annu Rev Plant Physiol 40:321–303
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li C-X, Feng S-L, Shao Y, Jiang L-N, Xu-yang L, Hou X-L (2007) Effects of arsenic on seed germination and physiological activities of wheat seedlings. J Environ Sci 19:725–32
Lindner RC (1944) Rapid analytical method for some of the more common organic substances of plant and soil. Plant Physiol 19:76–84
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Meharg AA, Macnair MR (1992) Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43:519–524
Meharg AA, Naylor J, Macnair MR (1994) Phosphorus nutrition of arsenate-tolerant and nontolerant phenotypes of velvet grass. J Environ Qual 23:234–238
Mengel K, Kirkby EA (2006) Principles of plant nutrition, 5th edn. Springer, The Netherlands, pp 439–447
Miteva E, Merakchiyska M (2002) Response of chloroplasts and photosynthetic mechanism of bean plants to excess arsenic in soil. Bulg J Agr Sci 8:151–156
Moreno-Jiménez E, Esteban E, Peñalosa JM (2012) The fate of arsenic in soil-plant systems. Rev Environ Contam Toxicol 215:1–37
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Narula A, Kumar S, Srivastava PS (2005) Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Reports 24:250–254
Nieboer E, Richardson DHS (1980) The replacement of the nondescript term “heavy metals” by a biologically significant and chemically significant classification of metal ions. Environ Pollut 1:3–26
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–27
Noctor G, Gomez LA, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, comparmentation, and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Odjegba VJ (2012) Exogenous salicylic acid alleviates arsenic toxicity in Arabidopsis thaliana. Indian J Innovation Develop 1:515–522
Penna LB, Tomaro ML, Gallego SM (2006) Effect of different metals on protease activity in sunflower cotyledons. Elect J Biotech 9:258–262
Poorter (1989) Plant growth analysis: towards a synthesis of the classical and functional approach. Physiol Plant 75:237–244
Saba PD, Iqbal M, Srivastava PS (2000) Effect of ZnSO4 and CuSo4 on regeration and Lepidine content in Lepidium sativum L. Biol Plant 43:253–256
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Sharma SS, Dietz KJ (2006) The significance of amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric oxide 20:289–297
Smith E, Naidu R, Alstron AM (2002) Chemistry of inorganic arsenic in soil effect of phosphorous, sodium, and calcium on arsenic absorption. J Environ Qual 3:557–563
Tu S, Ma LQ, MacDonald GE, Bondada B (2004) Effects of arsenic species and phosphorus on arsenic absorption, arsenate reduction, and thiol formation in excised parts of Pteris vittata L. Environ Exp Bot 51:121–131
Umar S (2006) Alleviating adverse effects of water stress on yield of sorghum, mustard, and groundnut by potassium application. Pak J Bot 38:1373–1380
Umar S, Diva I, Anjum NA, Iqbal M (2008) Potassium nutrition reduces cadmium accumulation and oxidative burst in mustard (Brassica campestris L.). Electronic Intl Fert Correspond 16:6–10
Umar S, Diva I, Anjum NA, Iqbal M, Ahmad I, Pereira E (2011) Potassium-induced alleviation of salinity stress in Brassica campestris L. Cent Eur J Biol 6:1054–1063
Urban PL, Bystrzejewska-Piotrowska G (2002) Comparative analysis of cesium and potassium uptake in onion Allium cepa L. Czech J Physics 52:A77–A82
Wahid A, Massod I, Javed I-u-H, Rasul E (1999) Phenotypic flexibility as marker of sodium chloride tolerance in sunflower genotypes. Environ Exp Bot 42:85–94
Wahid A (2007) Effect of cadmium on soil properties and plant growth. In: Bhatti SA, Arshad M (eds) Proceedings of national seminar on soil care for sustainable environment. Institute of Soil and Environmental sciences. University of agriculture, Faisalabad
White PJ, Broadley MR (2000) Mechanisms of caesium uptake by plants. New Phytol 147:241–256
Zhao FJ, Lombi E, McGrath SP (2003) Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 249:37–43
Zheng Y, Xu X, Simmons M, Li Z (2010) Responses of physiological parameters, grain yield and grain quality to foliar application of potassium nitrate in two contrasting winter wheat cultivars under salinity stress. J Plant Nutr Soil Sci 173:444–452
Acknowledgments
NG is grateful to Hillel Magen, Director, International Potash Institute (IPI) Switzerland, and to Potash Research Institute of India (PRII), Gurgaon, India for financial assistance. NAA is grateful to FCT (SFRH/BPD/64690/2009; SFRH/BPD/84671/2012) for the postdoctoral fellowship. Authors wish to acknowledge Jamia Hamdard (Hamdard University), New Delhi, India for providing required facilities to carry out the current research. Supports in terms of guidance, facilitating the experiment, and manuscript preparation kindly rendered by Drs. Deepshikha P. Katare and Arshi Anjum are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Umar, S., Gauba, N., Anjum, N.A. et al. Arsenic toxicity in garden cress (Lepidium sativum Linn.): significance of potassium nutrition. Environ Sci Pollut Res 20, 6039–6049 (2013). https://doi.org/10.1007/s11356-013-1624-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1624-y