Abstract
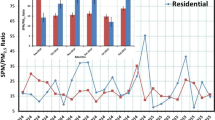
A Micro-Orifice Uniform Deposition Impactor (MOUDI) and a Nano-MOUDI were employed to determine the size-segregated mass distributions of ambient particulate matter (PM) and water-soluble ionic species for particulate constituents. In addition, gas precursors, including HCl, HONO, HNO3, SO2, and NH3 gases, were analyzed by an annular denuder system. PM size mass distribution, mass concentration, and ionic species concentration were measured during the day and at night during episode and non-episode periods in winter and summer. Average total suspended particle (TSP) concentrations during episode days in winter were as high as 153 ± 33 μg/m3, and PM mass concentrations in summer were as low as one-third of that in winter. Generally, PM concentration at night was higher than that in the daytime in southern Taiwan during the sampling periods. In winter during the episode periods, the size-segregated mass distribution of PM mass concentration was mostly in the 0.32–3.2-μm range, and the PM concentration increased significantly in the range of 0.32–3.2 μm at night. Ammonium, nitrate, and sulfate were the dominant water-soluble ionic species in PM, contributing 34–48 % of TSP mass. High concentrations of ammonia (12.9–49 μg/m3) and SO2 (2.6–27 μg/m3) were observed in the gas precursors. The conversion ratio was high in the PM size range of 0.18–3.2 μm both during the day and at night in winter, and the conversion ratio of episode days was 20 % higher than that of non-episode days. The conversion factor was high for both nitrogen and sulfur species at nighttime, especially on episode days.









Similar content being viewed by others
References
Behera SN, Sharma M (2011) Degradation of SO2, NO2 and NH3 leading to formation of secondary inorganic aerosols: an environmental chamber study. Atmos Environ 45:4015–4024
Calvert JG, Stockwell WR (1983) Acid generation in the troposphere by gas-phase chemistry. Environ Sci Technol 17:428–443
Calvert JG, Yarwood G, Dunker A (1994) An evaluation of the mechanism of nitrous acid formation in the urban atmosphere. Res Chem Intermed 20:463–502
Chang CT, Liu TH, Jeng FT (2004) Atmospheric concentrations of the Cl atom, CIO radical, and HO radical in the coastal marine boundary layer. Environ Res 94:67–74
Dongarrà G, Manno E, Varrica D, Lombardo M, Vultaggio M (2010) Study on ambient concentrations of PM10, PM10-2.5, PM2.5 and gaseous pollutants. Trace elements and chemical speciation of atmospheric particulates. Atmos Environ 44:5244–5257
Đorđevic D, Mihajlidi-Zelić A, Relić D, Ignjatović L, Huremović J, Stortini AM, Gambaro A (2012) Size-segregated mass concentration and water soluble inorganic ions in an urban aerosol of the central Balkans (Belgrade). Atmos Environ 46:309–317
Foltescu VL, Selin Lindgern E, Isakson J, Öblad M, Pacyna JM, Benson S (1996) Gas-to-particle conversion of sulfur and nitrogen compounds as studied at marine stations in northern European. Atmos Environ 30:3129–3140
Forster P, Ramaswamy V, Artaxo P, Bernsten T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga G, Schulz M, Van Dorland R (2007) Changes in atmospheric constituents and in radiative forcing. Cambridge, New York
Gao X, Yang L, Cheng S, Gao R, Zhou Y, Xue L, Shou Y, Wang J, Wang X, Nie W, Xu P, Wang W (2011) Semi-continuous measurement of water-soluble ions in PM2.5 in Jinan, China: temporal variations and source apportionments. Atmos Environ 45:6048–6056
Geller MD, Kim S, Misra C, Sioutas C, Olson BA, Marple VA (2002) A methodology for measuring size-dependent chemical composition of ultrafine particles. Aerosol Sci Technol 36:748–762
Glavas SD, Nikolakis P, Ambatzoglou D, Mihalopoulos N (2008) Factors affecting the seasonal variation of mass and ionic composition of PM2.5 at a central Mediterranean coastal site. Atmos Environ 42:5365–5373
Harrison RM, Kito AMN (1990) Field intercomparison of filter pack and denuder sampling methods for reactive gaseous and particulate pollutants. Atmos Environ 24:2633–2640
Harrison RM, Pio CA (1983) Size-differentiated composition of inorganic atmospheric aerosols of both marine and continental origin. Atmos Environ 17:1733–1738
Hegde P, Sudheer AK, Sarin MM, Manjunatha BR (2007) Chemical characteristics of atmospheric aerosols over southwest coast of India. Atmos Environ 41:7751–7766
Hidy GM (1994) Atmospheric sulfur and nitrogen oxides. Academic press, San Diego
Hitchcock DR, Spiller LL, Wilson WE (1980) Sulfuric acid aerosols and HCl release in coastal atmospheres: evidence of rapid formation of sulfuric acid particulates. Atmos Environ 14:165–182
Horemans B, Krata A, Buczynska AJ, Dirtu AC, Van Meel K, Van Grieken R, Bencs L (2009) Major ionic species in size-segregated aerosols and associated gaseous pollutants at a coastal site on the Belgian North Sea. J Environ Monitor 11:670–677
Hu JH, Abbatt JPD (1997) Reaction probabilities for N2O5 hydrolysis on sulfuric acid and ammonium sulfate aerosols at room temperature. J Phys Chem A 101:871–878
Huang XF, Li X, He LY, Feng N, Hu M, Niu YW, Zeng LW (2010) 5-Year study of rainwater chemistry in a coastal mega-city in South China. Atmos Res 97:185–193
Jenkin ME, Cox RA, Williams DJ (1988) Laboratory studies of the kinetics of formation of nitrous acid from the thermal reaction of nitrogen dioxide and water vapour. Atmos Environ 22:487–498
Kadowaki S (1977) Size distribution and chemical composition of atmospheric particulate nitrate in the Nagoya area. Atmos Environ 11:671–675
Keene WCPA (1986) Sea salt correction and interpretation of constituent ratios in marine precipitation. J Geophys Res 91:6647–6658
Khoder MI (2002) Atmospheric conversion of sulfur dioxide to particulate sulfate and nitrogen dioxide to particulate nitrate and gaseous nitric acid in an urban area. Chemosphere 49:675–684
Kittelson DB (1998) Engines and nanoparticles: a review. J Aerosol Sci 29:575–588
Kumar A, Sarin MM (2010) Atmospheric water-soluble constituents in fine and coarse mode aerosols from high-altitude site in western India: long-range transport and seasonal variability. Atmos Environ 44:1245–1254
Lee TY, Yu XY, Ayres B, Kreidenweis SM, Malm WC, Collett JL Jr (2008) Observations of fine and coarse particle nitrate at several rural locations in the United States. Atmos Environ 42:2720–2732
Li Y, An J, Min M, Zhang W, Wang F, Xie P (2011) Impact of HONO sources on the air quality in Beijing, Tianjin and Hebei Province of China. Atmos Environ 34:4711–4717
Lin YC, Cheng MT, Lin WH, Lan YY, Tsuang BJ (2010) Causes of the elevated nitrate aerosol levels during episodic days in Taichung urban area, Taiwan. Atmos Environ 44:1632–1640
Miguel AH, Eiguren-Fernandez A, Sioutas C, Fine PM, Geller M, Mayo PR (2005) Observation of twelve USEPA priority polycyclic aromatic hydrocarbons in the Aitken size range (10–32 nm Dp). Aerosol Sci Technol 39:415–418
Pathak RK, Yao XH, Chan LK (2004) Sampling artifacts of acidity and concentration of ionic species of PM2.5 in Hong Kong. Environ Sci Technol 38:254–259
Pathak RK, Wang T, Wu WS (2011) Nighttime enhancement of PM2.5 nitrate in ammonia-poor atmospheric conditions in Beijing and Shanghai: plausible contributions of heterogeneous hydrolysis of N2O5 and HNO3 partitioning. Atmos Environ 45:1183–1191
Perrino C, DeSantis F, Febo A (1990) Criteria for the choice of a denuder sampling technique devoted to the measurement of atmospheric nitrous and nitric acids. Atmos Environ 24A:617–626
Pio CA, Lopes DA (1998) Chlorine loss from marine aerosol in a coastal atmosphere. J Geophys Res 103:25263–25272
Plessow K, Spindler G, Zimmermann F, Matschullat J (2005) Seasonal variations and interactions of N-containing gases and particles over a coniferous forest, Saxony, Germany. Atmos Environ 39:6995–7007
Ravishankara A (1997) Heterogeneous and multiphase chemistry in the troposphere. Science 276:1058–1065
Russell AG, McRae GJ, Cass GR (1984) Acid deposition of photochemical oxidation products-a study using a lagrangian trajectory models. In: De Wispelaere C (ed) Air pollution modeling and its application III. Plenem, New York, pp 539–564
Russell AG, McRae GJ, Cass GR (1985) The dynamics of nitric acid production and the fate of nitrogen oxides. Atmos Environ 19:893–902
Sanhueza E (2001) Hydrochloric acid from chlorocarbons: a significant global source of background rain acidity. Tellus B 53:122–132
Seinfeld JH, Pandis SN (2006) Atmospheric chemistry and physics: from air pollution to climate change. Wiley, New York
Shi JP, Harrison RM, Brear F (1999) Particle size distribution from a modern heavy duty diesel engine. Sci Total Environ 235:305–317
Silva PJ, Liu DY, Noble CA, Prather KA (1999) Size and chemical characterization of individual particles resulting from biomass burning of local southern California species. Environ Sci Technol 33:3068–3076
Song CH, Nam JE, Han KM, Lee MK, Woo JH, Han JS (2011) Influence of mineral dust mixing-state and reaction probabilities on size-resolved sulfate formation in northern Asia. Atmos Environ 46:309–317. doi:10.1016/j.atmosenv.2011.10.057
Stelton AW, Seinfeld JH (1982) Relative humidity and temperature dependence of the ammonia nitrate dissociation constant. Atmos Environ 16:983–992
Stockwell WR, Watson JG, Robinson NF, Steiner W, Style WW (2000) The ammonium nitrate particle equivalent of NOx emissions for wintertime conditions in Central California's San Joaquin Valley. Atmos Environ 34:4711–4717
TEPA (Taiwan Environmental Protection Agency) (2005) Air pollutants emission database and air quality management program. EPA-94-FA11-030-A139. Taipei, Taiwan
TEPA (2012) Air quality annual report of Taiwan, ROC, 2011. htttp://www.epa.gov.tw
Thibert E, Dominé F (1997) Thermodynamics and kinetics of the solid solution of HCl in ice. J Phys Chem B 101:3554–3565
Tsai YI, Kou SC (2005) PM2.5 aerosol water content and chemical composition in a metropolitan and a coastal area in southern Taiwan. Atmos Environ 39:4827–4839
Tsai YI, Cheng MT (1999) Visibility and aerosol chemical compositions near the coastal area in central Taiwan. Sci Total Environ 231:37–51
US Environmental Protection Agency (USEPA) (1998) Compendium of methods for the determination of inorganic compounds in ambient air—IO-4.2 determination of reactive acid and basic gases and strong acidity in fine particles. USEPA publication 625/R-96/010a, Cincinnati
Vianaa AM, Pérezb C, Querola X, Alastueya A, Nickovicc S, Baldasano JM (2005) Spatial and temporal variability of PM levels and composition in a complex summer atmospheric scenario in Barcelona (NE Spain). Atmos Environ 39:5343–5361
Wakamatsu S, Utsunomiya A, Han JS, Mori A, Uno I, Uehara K (1996) Seasonal variation in atmospheric aerosol concentration covering Northern Kyushu, Japan and Seoul, Korea. Atmos Environ 30:2343–2354
Walker JT, Whitall DR, Robarge W, Paerl HW (2004) Ambient ammonia and ammonium aerosol across a region of variable ammonia emission density. Atmos Environ 38:1235–1246
Wall SM, John W, Ondo JL (1988) Measurement of aerosol size distributions for nitrate and major ionic species. Atmos Environ 22:1649–1656
Wang Y, Zhuang GS, Tang AH, Yuan H, Sun YL, Chen SA, Zheng AH (2005) The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos Environ 39:3771–3784
Zhang B, Tao FM (2010) Direction homogeneous nucleation of NO2, H2O and NH3 for the production of ammonium nitrate particles and HONO gas. Chem Phys Lett 489:143–147
Zhao J, Zhang F, Xu Y, Chen J (2011) Characterization of water-soluble inorganic ions in size-segregated aerosols in coastal city, Xiamen. Atmos Res 99:546–562
Acknowledgments
The authors acknowledge the support of National Science Council, Taiwan (NSC 94-2211-E-006-087, NSC 95-2211-E-006-287, and NSC 96-2211-E-006-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Stuart Simpson
Rights and permissions
About this article
Cite this article
Tsai, JH., Chang, LP. & Chiang, HL. Size mass distribution of water-soluble ionic species and gas conversion to sulfate and nitrate in particulate matter in southern Taiwan. Environ Sci Pollut Res 20, 4587–4602 (2013). https://doi.org/10.1007/s11356-012-1371-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1371-5