Abstract
Introduction
Understanding the sorption process in natural geomedia is necessary for effective utilization of these materials as low-cost adsorbents and consequently as controlled release hazardous elements. This research was oriented to investigate the affinity of two natural zeolite minerals towards cobalt, zinc, and nickel mixture as an important industrial and radioactive waste.
Method
The uptake of metal ions as a function of different parameters has been studied using a batch equilibrium technique.
Results
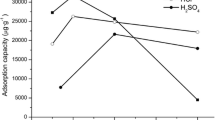
The results revealed that the affinity and adsorption capacity of chabazite and mordenite follow the order: Zn2+ > Co2+ > Ni2+, with good fits being obtained using Langmuir and Freundlich adsorption isotherms. The metal uptake was found to be concentration-dependent and independent of the pH over 3.0 to 8.0 range; this reveals that the adsorption mechanism is controlled mainly by a pure ion-exchange reaction at the experimental conditions used. Kinetic curves showed a rather fast exchange reaction for three cations, as equilibrium was mostly reached within 20 min.
Conclusion
These materials especially chabazite are recommended to be used as a reactive barrier for hazardous heavy metals control.






Similar content being viewed by others
References
Anirudhan TS, Suchithra PS (2010) Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: kinetics and equilibrium modeling. Chem Eng J 156:146–156
Arambula-Villazana V, Solache-Rios M, Olguin MT (2006) Sorption of cadmium from aqueous solutions at different temperatures by Mexican HEU-type zeolite rich tuff. J Incl Phenom Macrocycl Chem 55:237–245
Arellano F, Garcia-Sosa I, Solache-Rios (1995) Sorption of Co and Cd by zeolite Y. J. Radioanal. Nucl. Chem. Letters 199, 107–113
ATSDR (1992) Agency for toxic substances and disease registry. Toxicological profile for cobalt. Public Health Service, U.S. Department of Health and Human Services, Atlanta
Ayari F, Srasra E, Trabelsi-Ayadi M (2005) Characterization of bentonitic clays and their use as adsorbent. Desalination 185:391–397
Basha S, Murthy ZVP (2007) Kinetic and equilibrium models for biosorption of Cr(VI) on chemically modified seaweed, Cystoseira indica. Process Biochem 42:1521–1529
Borai EH, Harjula R, Malinen L, Paajanen A (2009) Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J Hazard Mater 172:416–422
Božić D, Stanković V, Gorgievski M, Bogdanović G, Kovačević R (2009) Adsorption of heavy metal ions by sawdust of deciduous trees. J Hazard Mater 171:684–692
Colella A, de Gennaro B (2001) Evaluation of Italian phillipsite and chabazite as cation exchangers for Ba2+ and Co2+. Stud Surf Sci Catal 140:153–162
Eccles H (1995) Removal of heavy metals from effluent streams—why select a biological process? Int Biodeterior Biodegradation 35:5–16
Eisler R (1993) Zinc hazard to fish, wildlife, and invertebrates: a synoptic review. (PDF). Contaminant hazard reviews. U.S. Department of the Interior, Fish and Wildlife Service, Laurel, Maryland, p 10
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Fosmire GJ (1990) Zinc toxicity. Am J Clin Nutr 51:225
Gustafsson JP (2009) www.lwr.kth.se/English/OurSoftware/vminteq/verhistory.htm
Hui KS, Chao CYH, Kot SC (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 127:89–101
Inglezakis VJ, Loizidou MD, Grigoropoulou HP (2002) Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res 36:2784–2792
Kasprzak (2003) Nickel carcinogenesis. Mutation Research 533: 67–97
Kosobucki P, Kruk M, Buszewski B (2008) Immobilization of selected heavy metals in sewage sludge by natural zeolites. Bioresour Technol 99:5972–5976
Lee S-H, Jo HY, Yun S-T, Lee YJ (2010) Evaluation of factors affecting performance of a zeolitic rock barrier to remove zinc from water. J Hazard Mater 175:224–234
Meena AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater 122:161–170
Mier MV, Callajas RL, Gehr R, Cisneros BEJ, Alvarez PJJ (2001) Heavy metal removal with Mexican clinoptilolite: multicomponentionic exchange. Water Res 35:373–378
Mondale KD, Carland RM, Aplan FF (1995) The comparative ion exchange capacities of natural sedimentary and synthetic zeolites. Miner Eng 8:535–548
Motsi T, Rowson NA, Simmons MJH (2009) Adsorption of heavy metals from acid mine drainage by natural zeolite. Int J Miner Process 92:42–48
Parab H, Joshi S, Shenoy N, Lali A, Sarma US, Sudersanan M (2006) Determination of kinetic and equilibrium of Co(II), Cr(III), and Ni(II) onto coir pith. Process Biochem 41:609–615
Peric J, Trgo M, Medvidovic NV (2004) Removal of zinc, copper and lead bynatural zeolite—a comparison of adsorption isotherms. Water Res 38:1893–1899
Serne RJ, Felmy AR, Cantrell KJ, Krupka KM, Campbell JA, Bolton H, Fredrickson JK (1995) Characterization of radionuclide-chelating agent complexes found in low-level radioactive decontamination waste: literature review. NUREG/CR-6124. U.S. Nuclear Regulatory Commission, Washington, D.C
Sheha RR (2007) Sorption behavior of Zn(II) ions on synthesized hydroxyapatites. J Colloid Interface Sci 310:18–26
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141
Sherry HS (1969) The ion exchange properties of zeolites. In: Marinsky JA (ed) Ion exchange. Marcel Dekker, New York, pp 2–89
Sheta AS, Falatah AM, Al-Sewailem MS, Khaled EM, Sallam ASH (2003) Sorption characteristics of zinc and iron by natural zeolite and bentonite. Microporous Mesoporous Mater 61:127–136
Shi W-yu, Shao, H.-bo, Li, H., Shao, M.-an, Du, S (2009) Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. Journal of Hazardous Materials 170: 1–6
Sprynskyy M, Buszewski B, Terzyk AP, Namiesnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J Colloid Interface Sci 304:21–28
Thyssen JP, Linneberg A, Menné T, Johansen JD (2007) The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermat 57:287–299
Trgo M, Perić J (2003) Interaction of the zeolitic tuff with Zn-containing simulated pollutant solutions. J Colloid Interface Sci 260:166–175
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24
Wang X-S, Huang J, Hu H-Q, Wang J, Qin Y (2007) Determination of kinetic and equilibrium parameters of the batch adsorption of Ni(II) from aqueous solutions by Na-mordenite. J Hazard Mater 142:468–476
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri.
Rights and permissions
About this article
Cite this article
Seliman, A.F., Borai, E.H. Utilization of natural chabazite and mordenite as a reactive barrier for immobilization of hazardous heavy metals. Environ Sci Pollut Res 18, 1098–1107 (2011). https://doi.org/10.1007/s11356-011-0459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0459-7