Abstract
Background, aim, and scope
Climate changes are nowadays an important issue of concern, and it is expected that in the near future it will be intensified, leading to extreme environmental conditions. These changes are expected to originate additional sources of stress; therefore, the exposure of organisms to natural stressors is receiving an increased importance in risk assessment. Organisms tend to avoid extremely environmental conditions looking for optimum conditions. This work aimed to evaluate the effects of natural stressors on the energetic reserves of Daphnia magna using the quantification of lipids, proteins, and sugars.
Materials and methods
Daphnids were exposed to different temperature regimes (16, 18, 22, 24, and 26°C), food levels (2, 1.5, 1, 0.5, and 0 and 4, 4.5, 5, 5.5, and 6 × 105 cells/ml Pseudokirchneriella subcapitata) and oxygen depletion (2 to 6 mg DO/L) and their energy reserves quantified. Protein, lipid, and sugar contents where compared between daphnids exposed to control conditions and ones exposed to considered stress situations.
Results and discussion
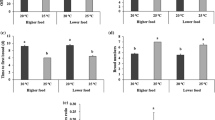
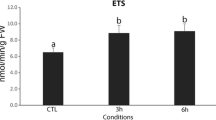
Significant changes in energy reserves content after a 96-h exposure were observed in temperatures 16, 22, 24, and 26°C. In the exposure to different food levels, daphnids showed significant differences on their energetic reserves when exposed to higher or lower levels of algae when compared with the control. Oxygen depletion did not affect significantly their energy budget.
Conclusions
The results from this work demonstrate that the environmental alterations related mainly to temperatures variations and food availability produced changes in D. magna energetic reserves. These changes can be transposed to the population levels as they are a result of changes in the metabolic rate and physiological processes that are related to growth and maturation.




Similar content being viewed by others
References
ASTM (1980) Standard practice for donducting acute toxicity tests with fishs, macroinvertebrates and amphibians, Report E-729-80. American Standards for Testing and Materials, Philadelphia
Baird DJ, Soares AMVM, Girling A, Barber I, Bradley MC, Calow P (1989) The long-term maintenance of Daphnia magna Straus for use in ecotoxicity tests: problems and prospects. In: Tyle H, Bro-Rasmussen F (eds) Proceedings of the first european conference on ecotoxicology Lokke H. Lyngby, Denmark, pp 144–148
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Canli M (2005) Dietary and water-borne Zn exposures affect energy reserves and subsequent Zn tolerance of Daphnia magna. Comp Biochem Physiol 141:110–116
De Coen WM, Janssen CR (1997) The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: a new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J Aquat Ecosyst Stress Recovery 6:43–55
De Coen WM, Janssen CR (1998) The use of biomarkers in Daphnia magna toxicity testing I. The digestive physiology of daphnids exposed to toxic stress. Hydrobiologia 367:199–209
De Coen WM, Vangheluwe ML, Janssen CR (1998) The use of biomarkers in Daphnia magna toxicity testing. IlL Rapid toxicity testing of pure chemicals and sediment pore waters using ingestion and digestive enzyme activity. Chemosphere 37:2677–2694
De Coen WM, Janssen CR, Segner H (2001) The use of biomarkers in Daphnia magna toxicity testing V. In vivo alterations in the carbohydrate metabolism of Daphnia magna exposed to sublethal concentrations of mercury and lindane. Ecotoxicol Environ Saf 48:223–234
Den Besten PJ (1998) Concepts for the implementation of biomarkers in environmental monitoring. Mar Environ Res 46:253–256
Ferreira ALG, Loureiro S, Soares AMVM (2008) Toxicity prediction of binary combinations of cadmium, carbendazim and low dissolved oxygen on Daphnia magna. Aquat Toxicol 89:28–39
Goulden CE, Hornig LL (1980) Population oscillations and energy reserves in planktonic cladocera and their consequences to competition. Proc Natl Acad Sci U S A 77:1716–1720
Hanazato T, Dodson SI (1995) Synergistic effects of low oxygen concentration, predator kairomone, and a pesticide on the cladoceran Daphnia pulex. Limnol Oceanogr 40:700–709
Holmstrup M, Sørensen LI, Bindesbøl AM, Hedlund K (2007) Cold acclimation and lipid composition in the earthworm Dendrobaena octaedra. Comp Biochem Physiol 147:911–919
IPCC (2007) Painel Intergovernamental sobre Mudança do Clima. OMM/PNUMA, Genebra
Lagerspetz KYH (2000) Thermal avoidance and preference in Daphnia magna. J Therm Biol 25:405–410
Mayer FL, Versteeg DJ, McKee MJ, Folmar LC, Graney RL, McCume DC, Rattner BA (1992) Physiological and nonspecific biomarkers, biomarkers. biochemical, physiological, and histological markers of anthropogenic stress. Lewis Publishers, Boca Raton, p 85
Mourelatos S, Lacroix G (1990) In situ filtering rates of Cladocera: effect of body lenght, temperature, and food concentration. Limnol Oceanogr 35:1101–1111
OECD (1998) Daphnia magna reproduction test. OECD 211. OECD—Organization for Economic Cooperation and Development, Paris
OECD (2000) Daphnia sp., acute immobilisation test. OECD 202. OECD—Organization for Economic Cooperation and Development, Paris
Overgaard J, Tomcala A, Sørensen JG, Holmstrup M, Krogh PH, Simek P, Kostal V (2008) Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J Insect Physiol 54:619–629
Pieters BJ, Liess M (2006) Maternal nutritional state determines the sensitivity of Daphnia magna offspring to short-term Fenvalerate exposure. Aquat Toxicol 76:268–277
Rinke K, Petzoldt T (2003) Modelling the effects of temperature and food on individual growth and reproduction of Daphnia and their consequences on the population level. Limnologica 33:293–304
Rinke K, Vijverberg J (2005) A model approach to evaluate the effect of temperature and food concentration on individual life-history and population dynamics of Daphnia. Ecol Modell 186:326–344
Seidl MD, Pirow R, Paul RJ (2005) Acclimation of the microcrustacean Daphnia magna to warm temperatures is dependent on haemoglobin expression. J Therm Biol 30:532–544
Simcic T, Brancelj A (1997) Electron transport system (ETS) activity and respiration rate in five Daphnia species at different temperatures. Hydrobiologia 360:117–125
Simcic T, Brancelj A (2001) Seasonal dynamics of metabolic activity of the Daphnia community in Lake Bled (Slovenia). Hydrobiologia 442:319–328
Vezjak M, Savsek T, Stuhler EA (1998) System dynamics of euthrophication processes in lakes. Eur J Oper Res 109:442–451
Wiggins PR, Frappell PB (2002) Behavioural thermoregulation in Daphnia carinata from different depths of a natural water body: influence of environmental oxygen levels and temperature. Comp Biochem Physiol 133:771–780
Zar JH (1996) Biostatistical analysis. Prentice Hall, Upper Saddle River, p 662
Zeis B, Lamkemeyer T, Paul RJ (2004) Molecular adaptation of Daphnia magna hemoglobin. Micron 35:47–49
Acknowledgments
The study was supported by the EU Integrated project NoMiracle (Novel Methods for Integrated Risk assessment of Cumulative Stressors in Europe; http://nomiracle.jrc.it) contract No. 003956 under the theme under the EU-theme “Global Change and Ecosystems” topic “Development of risk assessment methodologies”, coordinated by Dr. Hans Løkke at NERI, DK-8600 Silkeborg, Denmark. The Programme Alban, the European Union Programme of High Level Scholarships for Latin America, supported Tullus U. Bergman Filho with a M.Sc. grant (E07M402154BR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Henner Hollert
Rights and permissions
About this article
Cite this article
Bergman Filho, T.U., Soares, A.M.V.M. & Loureiro, S. Energy budget in Daphnia magna exposed to natural stressors. Environ Sci Pollut Res 18, 655–662 (2011). https://doi.org/10.1007/s11356-010-0413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0413-0