Abstract
Purpose
Adsorption of metals (Pb, Cd, Cu, Ni, Zn) to TiO2 nanoparticles and bulk particles was examined for use as a contaminant removal substrate as a function of particle size, sorbent concentration, and exhaustion.
Methods
Adsorption experiments were conducted with 0.01, 0.1, and 0.5 g/L nanoparticles in a pH 8 solution and in spiked San Antonio tap water.
Results
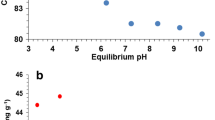
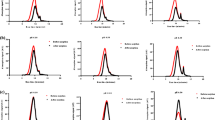
When results were normalized by mass, nanoparticles adsorbed more than the bulk particles but when results were surface-area normalized, the opposite was observed. The adsorption data shows the ability of the TiO2 nanoparticles to remove Pb, Cd, and Ni from solution with similar adsorption at 0.1 and 0.5 g/L. Adsorption kinetics for all metals tested was described by a modified first order rate equation with the nanoparticles having a faster rate of adsorption than the bulk particles. The nanoparticles were able to simultaneously removal multiple metals (Zn, Cd, Pb, Ni, Cu) from both pH 8 solution and spiked San Antonio tap water. Exhaustion experiments showed that both the nanoparticles and bulk particles were exhausted at pH 6 but at pH 8, exhaustion did not occur for the nanoparticles.
Conclusion
Comparison of K d, distribution coefficient, with other literature showed that the nanoparticles were better sorbents than other metal oxide nanoparticles and a commercial activated carbon.





Similar content being viewed by others
References
Adesina (2004) Industrial exploitation of photocatalysis progress, perspectives and prospects. Catal Surv Asia 8:265–273
Auffan M (2007) In environmental nanotechnology. McGraw-Hill Publishing, New York, p 540
Banfield JF, Navrotsky A (2003) Nanoparticles and the environment. Reviews in mineralogy and geochemistry. Geochimica et Cosmochimica Acta, 67, Washington DC, p 1753
Brunauer S, Emmett P, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309
Chitose N, Ueta S, Yamamoto T (2003) Radiolysis of aqueous phenol solutions with nanoparticles 1. phenol degradation and TOC removal in solutions containing TiO2 induced by UV, gamma-ray and electron beams. Chemosphere 50:1007–1013
Dutta P, Ray A, Sharma V, Millero J (2004) Adsorption of arsenate and arsenite on titanium dioxide suspensions. J Colloid Interface Sci 278:270–275
Dzombak D, Morel F (1990) Surface complexation modeling: hydrous ferric oxide. Wiley, New York, p 393
El-Sayed MA (2001) Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res 34:257–264
Giammar DE, Maus CJ, Xie LY (2007) Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles. Environ Eng Sci 24:85–95
Glazier R, Venkatakrishnan R, Gheorghiu F, Walata L, Nash R, Zhang W (2003) Nanotechnology takes root. Civ Eng 73:64–69
Guzman KA, Finnegan MP, Banfield JF (2006) Influence of surface potential on aggregation and transport of titania nanoparticles. Environ Sci Technol 40:7688–7693
HACHCompany (2008) HACH water analysis handbook procedures. HACH, Loveland
Hu J, Chen G, Lo I (2006) Selective removal of heavy metals from industrial wastewater using maghemite nanoparticle: performance and mechanisms. J Environ Eng 132:709–715
Kabra K, Chaudhary R, Sawhney R (2004) Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: a review. Ind Eng Chem Res 43:7683–7696
Kosmulski M (2002) The significance of the difference in the point of zero charge between rutile and anatase. Adv Colloid Interface Sci 99:255–264
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I: solids. J Am Chem Soc 38:2221–2295
Liang P, Shi T, Li J (2004) Nanometer-size titanium dioxide separation/preconcentration and FAAS determination of trace Zn and Cd in water sample. Int J Environ Anal Chem 84:315–321
Lisha KP, Anshup, Pradeep T (2009) Towards a practical solution for removing inorganic merucry from drinking water using gold nanoparticles. Gold Bulletin, pp 144–152
Liu W-T (2006) Nanoparticles and their biological and environmental applications. J Biosci Bioeng 102:1–7
Martra A (2000) Lewis acid and base sites at the surface of microcrystalline Ti 02 anatase: relationships between surface morphology and chemical behaviour. Appl Catal 200:275–285
Sa M (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, New York, p 1024
Morterra C (1988) An infrared spectroscopic study of antase properties Part 6. J Chem Soc, Faraday Trans 84:1617–1622
Rebhun M, Galil N (1990) Wastewater treatment technologies. In: Zirm L, Mayer J (eds) The management of hazardous substances in the environment. Elsevier Applied Science, London, pp 85–102
Savage N, Diallo M (2005) Nanomaterials and water purification: opportunities and challenges. J Nanopart Res 7:331–342
Shipley HJ, Yean S, Kan AT, Tomson MB (2009) Adsorption of arsenic to magnetite nanoparticles: effect of particle concentration, pH, ionic strength, and temperature. Environ Toxicol Chem 28:509–515
Stumm W, Morgan J (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, New York, p 1024
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nano Today, pp 44–48
UNEPDEWA/GRID-Europe (2005) E-waste, the hidden side of IT equipment's manufacturing and use http://www.grid.unep.ch/
US EPA (2006) 2006 Edition of the Drinking water standards and health advisories. In: Water Oo (Hrsg.), Washington, D.C.
Waychunas G, Kim C, Banfield J (2005) Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms. J Nanopart Res 7:409–433
WHO (2005) Nickel in drinking water: background document for development of WHO Guidelines for drinking water quality
Xu Z, Liu X, Ma Y, Gao H (2010) Interaction of nano-TiO2 with lysozyme: insights into the enzyme toxicity of nanosized particles. Environ Sci Pollut Res 17:798–806
Yantasee W, Warner CL, Sangvanich T, Addleman RS, Carter TG, Wiacek RJ, Fryxell GE, Timchalk C, Warner MG (2007) Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ Sci Technol 41:5114–5119
Acknowledgments
We acknowledge financial support from the National Science Foundation through the Broadening Participation Research Initiation Grant in Engineering (EEC-0823685) and the University of Texas at San Antonio.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Engates, K.E., Shipley, H.J. Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: effect of particle size, solid concentration, and exhaustion. Environ Sci Pollut Res 18, 386–395 (2011). https://doi.org/10.1007/s11356-010-0382-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0382-3