Abstract
Background, aim, and scope
Phenols are the most common pollutants in industrial wastewaters (particularly from oil refineries, resin manufacture, and coal processing). In the last two decades, it has become common knowledge that they can be effectively destroyed by nonconventional techniques such as power ultrasound (US) and/or microwave (MW) irradiation. Both techniques may strongly promote advanced oxidation processes (AOPs). The present study aimed to shed light on the effect and mechanism of US- and MW-promoted oxidative degradation of chlorophenols; 2,4-dichlorophenoxyacetic acid (2,4-D), a pesticide widespread in the environment, was chosen as the model compound.
Materials and methods
2,4-D degradation by AOPs was carried out either under US (20 and 300 kHz) in aqueous solutions (with and without the addition of Fenton reagent) or solvent-free under MW with sodium percarbonate (SPC). All these reactions were monitored by gas chromatography–mass spectrometry (GC–MS) analysis and compared with the classical Fenton reaction in water under magnetic stirring. The same set of treatments was also applied to 2,4-dichlorophenol (2,4-DCP) and phenol, the first two products that occur a step down in the degradation sequence. Fenton and Fenton-like reagents were employed at the lowest active concentration.
Results
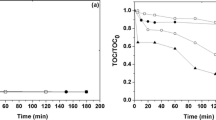
The effects of US and MW irradiation were investigated and compared with those of conventional treatments. Detailed mechanisms of Fenton-type reactions were suggested for 2,4-D, 2,4-DCP, and phenol, underlining the principal degradation products identified. MW-promoted degradation under solvent-free conditions with solid Fenton-like reagents (viz. SPC) is extremely efficient and mainly follows pyrolytic pathways. Power US strongly accelerates the degradation of 2,4-D in water through a rapid generation of highly reactive radicals; it does not lead to the formation of more toxic dimers.
Discussion
We show that US and MW enhance the oxidative degradation of 2,4-D and that a considerable saving of oxidants and cutting down of reaction times is thereby achieved. The results support the interpretation of previously published data and improve the understanding of the factors of direct degradation along different pathways.
Conclusions
Oxidative pathways for 2,4-D, 2,4-DCP, and phenol were proposed by a careful monitoring of the reactions and detection of intermediates by GC–MS.
Recommendations and perspectives
The understanding of the factors that affect chlorophenols degradation along different pathways may facilitate the optimization of the treatment. Type of energy source (US or MW), power, and frequency to be applied could be designed in function of the operative scenario (amount of pollutant in soil, water, or oils).






Similar content being viewed by others
References
Abramovitch RA, Michael C (2003) Remediation of waters contaminated with pentachlorophenol. Chemosphere 50:955–957
Agarry SE, Durojaiye AO, Solomon BO (2008) Microbial degradation of phenols: a review. Int J Environ Pollut 32:12–28
Ai ZH, Yang P, Lu XH (2005) Degradation of 4-chlorophenol by microwave irradiation enhanced advanced oxidation processes. Chemosphere 60:824–827
Aly OM, Faust SD (1964) Herbicides in surface waters, studies on fate of 2, 4-D and ester derivatives in natural surface waters. J Agric Food Chem 12:541–546
Antunes CS, Bietti M, Ercolani G, Lanzalunga O, Salamone M (2005) The effect of ring substitution on the O-neophyl rearrangement of 1, 1-diarylalkoxyl radicals. A product and time-resolved kinetic study. J Org Chem 70:3884–3891
Australian Government, Department of the Environment, Water, Heritage and the Arts, The National Pollutant Inventory (NPI) (2008). Available at http://www.npi.gov.au/database/substanceinfo/profiles/70.html
Barrault J, Abdellaoui M, Bouchoule C, Majesté A, Tatiboüet JM, Laulodi A, Papayannakos N, Gangas NH (2000) Mechanism of the oxidation of aqueous phenol with dissolved oxygen. Appl Catal B Environ 27:225–230
Berlan J, Trabelsi F, Delmas H, Wilhelm AM, Petrignan JF (1994) Pulsed sonolysis of surfactants in aqueous solutions. Ultrason Sonochem 1:S97–S102
Bhargava SK, Tardio J, Prasad J, Foger K, Akolekar DB, Grocott SC (2006) Wet oxidation and catalytic wet oxidation. Ind Eng Chem Res 45:1221–1258
Bremner D, Di Carlo S, Chakinala AG, Cravotto G (2008) Mineralisation of 2, 4-dichlorophenoxyacetic acid by acoustic or hydrodynamic cavitation in conjunction with the advanced Fenton process. Ultrasonics Sonochemistry 15:416–419
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160:265–288
Buxton G, Greenstock C, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Phys Chem Ref Data 17:513–886
Cravotto G, Di Carlo S, Tumiatti V, Roggero C, Bremner D (2005) Degradation of persistent organic pollutants by Fenton’s reagent facilitated by microwave or high-intensity ultrasound. Environ Technol 26:721–724
Cravotto G, Di Carlo S, Curini M, Tumiatti V, Roggero C (2007a) A new flow reactor for the treatment of polluted water with microwave and ultrasound. J Chem Tech Biotech 82:205–208
Cravotto G, Ondruschka B, Di Carlo S, Roggero C, Tumiatti V (2007b) Decontamination of soil containing POPs by the combined action of solid Fenton-like reagents and microwaves. Chemosphere 69:1326–1329
Cravotto G, Di Carlo S, Mantegna S, Binello A, Girlanda M, Lazzari A (2008) Integrated sonochemical and microbial treatment for decontamination of nonylphenol-polluted water. Water Air Soil Pollut 187:353–359
Destaillats H, Colussi AJ, Joseph JM, Hoffmann MR (2000) Synergistic effects of sonolysis combined with ozonolysis for the oxidation of azobenzene and methyl orange. J Phys Chem A 104:8930–8935
Devlin HR, Harris IJ (1984) Mechanism of the oxidation of aqueous phenol with dissolved oxygen. Ind Eng Chem Fundam 23:387–392
Du Y, Zhou M, Lei L (2007) Kinetic model of 4-CP degradation by Fenton/O2 system. Water Res 41:1121–1133
Falvey DE, Khambatta BS, Schuster G (1990) Neophyl-like rearrangement of alkoxy radicals direct detection of a bridged intermediate by time-resolved absorption-spectroscopy. J Phys Chem 94:1056–1059
Gimeno O, Carbajo M, Beltran FJ, Rivas FJ (2005) Phenol and substituted phenols AOPs remediation. J Hazard Mater 119:99–108
Gogate PR, Pandit BA (2004) Review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Adv Environ Res 8:501–551
Gopalan S, Savage PE (1994) Reaction mechanism for phenol oxidation in supercritical water. J Phys Chem 98:12646–12652
Goskonda S, Catallo WJ, Junk T (2002) Sonochemical degradation of aromatic organic pollutants. Waste Management 22:351–356
Hao H, Chen Y, Wu M, Wang H, Yin Y, Lü Z (2004) Sonochemistry of degrading p-chlorophenol in water by high frequency ultrasound. Ultrason Sonochem 11:43–46
Higashimura H, Fujisawa K, Kubota M, Kobayashi S (2005) “Radical-controlled” oxidative polymerization of phenol: comparison with that of 4-phenoxyphenol. J Polym Sci Part A Polym Chem 43:1955–1962
Jiang Y, Waite TD (2003) Degradation of trace contaminants using coupled sonochemistry and Fenton’s reagent. Water Sci Technol 47:85–92
Jones CW (1999) Applications of hydrogen peroxide and derivatives. Royal Society of Chemistry, Cambridge p 264. doi:10.1021/op000003w
Joseph JM, Destaillats H, Hung HM, Hoffmann MR (2000) The sonochemical degradation of azobenzene and related azo dyes: rate enhancements via Fenton’s reactions. J Phys Chem A 104:301–307
Kaioumova D, Kaioumov F, Opelz G, Susal C (2001) Toxic effects of the herbicide 2, 4-dichlorophenoxyacetic acid on lymphoid organs of the rat. Chemosphere 43:801–805
Kang JW, Hung HM, Alin A, Hoffmann MR (1999) Sonolytic destruction of methyl tert-buthyl ether by ultrasonic irradiation: the role of O3, H2O2, frequency, and power density. Environ Sci Technol 33:3199–3205
Kidak R, Ince NH (2006) Ultrasonic destruction of phenol and substituted phenols: a review of current research. Ultrason Sonochem 13:195–199
Lesko T, Colussi AJ, Hoffmann MR (2006) Sonochemical decomposition of phenol: evidence for a synergistic effect of ozone and ultrasound for the elimination of total organic carbon from water. Environ Sci Technol 40:6818–6823
Li X, Cubbage JW, Jenks WS (1999) Photocatalytic degradation of 4-chlorophenol. 2. The 4-chlorocatechol pathway. J Org Chem 64:8525–8536
Liang J, Komarov S, Hayashi N, Kasai E (2007) Improvement in sonochemical degradation of 4-chlorophenol by combined use of Fenton-like reagents. Ultrason Sonochem 12:201–207
Liotta LF, Gruttadauria M, Di Carlo G, Perrini G, Librando V (2009) Heterogeneous catalytic degradation of phenolic substrates: catalysts activity. J Haz Mat 162:588–606
Mason TJ (1992) Industrial sonochemistry: potential and practicality. Ultrasonics 30:192–196
Matos J, Laine J, Hermann JM (2001) Effect of the type of activated carbons on the photocatalytic degradation of aqueous organic pollutants by UV-irradiated titania. J Catal 200:10–20
Menéndez JA, Inguanzo M, Pis JJ (2002) Microwave-induced pyrolysis of sewage sludge. Water Res 36:3261–3264
Papadaki M, Emery RJ, Abu-Hassan MA, Díaz-Bustos A, Metcalfe IS, Mantzavinos D (2004) Sonocatalytic oxidation processes for the removal of contaminants containing aromatic rings from aqueous effluents. Sep Purific Technol 34:35–42
Peller J, Wiest O, Kamat PV (2001) Sonolysis of 2, 4-dichlorophenoxyacetic acid in aqueous solutions. Evidence for •OH-radical-mediated degradation. J Phys Chem A 105:3176–3181
Petrier C, Jiang Y, Lamy MF (1998) Ultrasound and environment: sonochemical destruction of chloroaromatic derivative. Environ Sci Technol 32:1316–1318
Polaert I, Estel L, Ledoux A (2005) Microwave assisted remediation of phenol wastewater on activated charcoal. Chem Engin Sci 60:6354–6359
Riesz P, Kondo T (1992) Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med 13:247–270
Stavarache C, Yim B, Vinatoru M, Maeda Y (2002) Sonolysis of chlorobenzene in Fenton-type aqueous systems. Ultrason Sonochem 9:291–296
Song Q, Niu T, Wang H (2008) Theoretical study of the reaction of 2, 4-dichlorophenol with 1O2. J Mol Struct (Theochem) 861:27–32
Tauber A, Schuchmann HP, Von Sonntag C (2000) Sonolysis of aqueous 4-nitrophenol at low and high pH. Ultrason Sonochem 7:45–52
Uyama H, Maruichi N, Tonami H, Kobayashi S (2002) Peroxidase-catalyzed oxidative polymerization of bisphenols. Biomacromolecules 3:187–193
Weavers LK, Ling FK, Hoffmann MR (1998) Aromatic compound degradation in water using a combination of sonolysis and ozonolysis. Environ Sci Technol 32:2727–2733
Wu ZL, Ondruschka B, Cravotto G (2008) Degradation of phenol under combined irradiation of microwaves and ultrasound. Environ Sci Technol 42:8083–8087
Yasman Y, Bulatov V, Gridin V, Agur S, Galil N, Armon R, Schechter I (2004) A new sono-electrochemical method for enhanced detoxification of hydrophilic chloroorganic pollutants in water. Ultrason Sonochem 11:365–372
Yu J, Taylor KE, Zou H, Biswas N, Bewtra JK (1994) Phenol conversion and dimeric intermediates in horseradish peroxidase-catalyzed phenol removal from water. Environ Sci Technol 28:2154–2160
Yu Y, Ma J, Hou YJ (2006) Degradation of 2, 4-dichlorophenoxyacetic acid in water by ozone-hydrogen peroxide process. J Environ Sci 18:1043–1049
Zazo JA, Casas JA, Mohedano AF, Gilarranz MA, Rodríguez JJ (2005) Chemical pathway and kinetics of phenol oxidation by Fenton's reagent. Environ Sci Technol 39:9295–9302
Zhihui A, Peng Y, Xiaohua L (2005) Degradation of 4-chlorophenol by microwave irradiation enhanced advanced oxidation processes. Chemosphere 60:824–882
Acknowledgment
This work was carried out under the auspices of COST Action D32 (WGs 06 and 08). Authors thank the University of Torino for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Cravotto, G., Binello, A., Di Carlo, S. et al. Oxidative degradation of chlorophenol derivatives promoted by microwaves or power ultrasound: a mechanism investigation. Environ Sci Pollut Res 17, 674–687 (2010). https://doi.org/10.1007/s11356-009-0253-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0253-y