Abstract
Background, aim, and scope
Polysaccharides are renewable resources representing an important class of polymeric materials of biotechnological interest, offering a wide variety of potentially useful products to mankind. Exopolysaccharides (EPSs) of microbial origin with a novel functionality, reproducible physico-chemical properties, stable cost and supply, became a better alternative to polysaccharides of algal origin. EPSs are believed to protect bacterial cells from desiccation, heavy metals or other environmental stresses, including hostimmune responses, and to produce biofilms, thus enhancing the cells chances of colonising special ecological niches. One of the most important stress factor is salt stress for microorganisms. The present investigation is aimed to determine correlation between salt resistance and EPS production by three cyanobacterial isolates (Synechocystis sp. BASO444, Synechocystis sp. BASO507 and Synechocystis sp. BASO511). It is also aimed to investigate the effect of salt concentrations on EPS production by cyanobacteria and effect of salt on monosaccharide composition of EPS.
Materials and methods
Cyanobacterial isolates were identified by 16 S rRNA analysis. Its salt (NaCl) tolerance and association with exopolysaccharides (EPSs) production in three cyanobacterial isolates were investigated. Also, EPS was analysed by HPLC for monomer characterization.
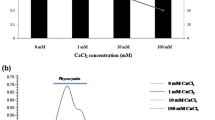
Results
Increased EPS production was associated with NaCl tolerance. The most tolerant isolate, Synechocystis sp. BASO444, secreted the most EPS (500 mg/L). EPS production by Synechocystis sp. BASO444, Synechocystis sp. BASO507 and Synechocystis sp. BASO511 was investigated following exposure to 0.2 and 0.4 M NaCl. Also, flasks containing medium without NaCl were inoculated in the same manner to serve as controls. The monosaccharide compositions of EPS produced by the three isolates following exposure to 0.2 M NaCl were analysed by HPLC. Control EPS of BASO444 was composed of glucose (97%) and galacturonic acid (3%). The composition of BASO511 (control) was glucose (95%), xylose (4.80%), arabinose (0.13%), glucuronic acid (0.03%) and galacturonic acid (0.04%). However, the composition of BASO507 (control) was glucose (0.98%), xylose (98.00%), arabinose (1.00%), glucuronic acid (0.01%) and galacturonic acid (0.01%). In the presence of 0.2 M NaCl, EPS compositions and ratios of three cyanobacterial isolates changed.
Discussion
Although hyperproduction of EPS in response to starvation, antiviral activity, thickening agent and cosmetic industry for product formulations has been reported for cyanobacteria, the effect of NaCl on EPS production in cyanobacteria is not a popular area of study. There are no clear reports correlating EPS production and NaCl tolerance. The gap in the data about the effect of NaCl on cyanobacterial EPS production was filled by this investigation, and the results of our study have important implications in both the industrial and environmental arenas.
Conclusions
Our results indicate that 1) exposure to elevated concentrations of NaCl affects the composition of EPS produced by Synechocystis sp. BASO444, Synechocystis sp. BASO507 and Synechocystis sp. BASO511, and 2) there is a correlation between NaCl tolerance and EPS production in some cyanobacteria.
Recommendations and perspectives
Differences in the monosaccharide composition and ratios of EPS may promote NaCl tolerance in these microorganisms. As well, these alternative composition polysaccharides may be important for industrial applications.


Similar content being viewed by others
References
Aquino SF, Stuckey DC (2002) Characterization of soluble microbial products (SMP) in effluents from anaerobic reactors. Water Sci Technol 45(10):127–132
Bar-Or Y, Shilo M (1987) Characterization of macromolecular flocculants produced by Phormidium sp. strain J-1 and by Anabaenopsis circularis PCC6720. Appl Environ Microb 53:2226–2230
Bender J, Phillips P (2004) Microbial mats for multiple applications in aquaculture and bioremediation. Bioresour Technol 94:229–238
Borrok DM, Fein JB (2005) The impact of ionic strength on the adsorption of protons, Pb, Cd, and Sr onto the surfaces of Gram negative bacteria: testing non-electrostatic, diffuse, and triple-layer models. J Colloid Interface Sci 286:110–126
Brown AD (1990) Microbial water stress physiology. Principles and perspectives. Wiley, New York
Brüll LP, Huang ZB, Thomas-Oates JE, Paulsen BS, Cohen EH, Michaelsen TE (2000) Studies of polysaccharides from three edible species of Nostoc (cyanobacteria) with different colony morphologies: structural characterization and effect on the complement system of polysaccharides from Nostoc commune. J Phycol 36:871–881
Cérantola S, Bounéry JD, Segonds C, Marty N, Montrozier H (2000) Exopolysaccharide production by mucoid and non-mucoid strains of Burkholderia cepacia. FEMS Microbiol Lett 185:243–246
Chen L-Z, Li D-H, Song L-R, Hu C-X, Wang G-H, Liu Y-D (2006) Effects of salt stress on carbohydrate metabolism in desert soil alga Microcoleus vaginatus Gom. J Integr Plant Biol 48:914–919
Chi Z, Fang Y (2005) Exopolysaccharides from marine bacteria. J Ocean Univ China 14:67–74
Claessens J, Van Cappellen P (2007) Competitive binding of Cu2 and Zn2+ to live cells of Schewanella putrefaciens. Environ Sci Technol 41:909–914
Claessens J, van Lith Y, Laverman AM, Van Cappellen P (2006) Acid-base activity of live bacteria: implications for quantifying cell wall charge. Geochim Cosmochim Acta 70:267–276
Davey ME, O'Toole GA (2000) Molecular biofilms: from ecology to molecular genetics. Microbiol Mol Biol R 64:847–867
De Philippis R, Vincenzini M (1998) Exocellular polysacharides from cyanobacteria and their possible applications. FEMS Microbiol Rev 22:151–171
De Philippis R, Sili C, Paperi R, Vincenzini M (2001) Exopolysaccharide-producing cyanobacteria and their possible exploitation. J Appl Phycol 13:293–299
Dittrich M, Sibler S (2006) Influence of H+ and calcium ions on surface functional groups of Synechococcus PCC 7942 cells. Langmuir 22:5435–5442
Dittrich M, Muller B, Mavrocordatos D, Wehrli B (2003) Induced calcite precipitation by cyanobacterium Synechococcus. Acta Hydrochim Hydrobiol 31:162–169
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric methods for determination of sugars and related substances. Anal Chem 28:350–356
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge
Freire-Nordi CS, Vieira AAH, Nascimento OR (2005) The metal binding capacity of Anabaena spiroides extracellular polysaccharide: an EPR study. Process Biochem 40:2215–2224
Graham LE, Wilcox LW (2000) Algae. Prentice-Hall, Englewood Cliffs
Hirabayashi J (2003) Oligosaccharide microarrays for glycomics. Trends Biotechnol 21:141–143
Hirschberg J, Chamovitz D (1994) Carotenoids in cyanobacteria. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer, Dordrecht, pp 559–579
Hu C, Liu Y, Paulsen BS, Petersen D, Klaveness D (2003) Extracellular carbohydrate polymers from five desert soil algae with different cohesion in the stabilization of fine sand grain. Carbohyd Polym 54:33–42
Kazy SK, Sar P, Singh SP, Sen AK, D’Souza SF (2002) Extracellular polysaccharides of a copper-sensitive and copper-resistant Pseudomonas aeruginosa strain: synthesis, chemical nature and copper binding. World J Microbiol Biotechnol 18:583–588
Liu H, Buskey EJ (2000) Hyper salinity enhances the production of extracellular polymeric substance (EPS) in the texas brown tide alga, Aureoumbra lagunensis (Pelagophyceae). Journal Phycol 36:71–77
Lloret J, Wulff BB, Rubio JM, Downie JA, Bonilla I, Rivilla R (1998) Exopolysaccharide II production is regulated by salt in the halotolerant strain Rhizobium meliloti EFB1. Appl Environ Microbiol 64:1024–1028
Mackay MA, Norton RS, Borowitzka LJ (1984) Organic osmoregulatory solutes in cyanobacteria. J Gen Microb 130:2177–2191
Martinez RE, Smith DS, Kulczycki E, Ferris FG (2002) Determination of intrinsic bacterial surface acidity constants using a Donnan shell model and a continuous pK a distribution method. J Colloid Interface Sci 253:130–139
Moreno J, Vargas MA, Madiedo JM, Munoz J, Rivas J, Guerrero MG (2000) Chemical and rheological properties of extracellular polysaccharide produced by the cyanobacterium Anabaena sp. ATCC 33047. Biotechnol Bioeng 67:283–290
Nicolaus B, Panico A, Lama L, Romano I, Manca MC, Giulio AD, Gambacorta A (1999) Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyanobacteria. Phytochem 52:639–647
Nubel U, Garcia-Pichel F, Muyzer G (1997) PCR primers to amplify 16 S rRNA genes from cyanobacteria. Appl Environ Microbiol 63:3327–3332
Obst M, Dittrich M, Kuehn H (2006) Calcium adsorption and changes of the surface microtopography of cyanobacteria studied by AFM, CFM, and TEM with respect to biogenic calcite nucleation. Geochem Geophys Geosystems G3 7 10.1029/2005GC001172
Ozturk S, Aslim B (2008) Relationship between chromium(VI) resistance and extracellular polymeric substances (EPS) concentration by some cyanobacterial isolates. Environ Sci Pollut Res 15(6):478–480
Ozturk S, Aslim B, Ugur A (2008) Chromium(VI) resistance and extracellular polysaccharide (EPS) synthesis by Pseudomonas, Stenotrophomonas and Methylobacterium strains. ISIJ Int 48(11):1654–1658
Pokrovsky OS, Kompantseva EI (2007) Experimental physicochemical modeling of interactions between phototrophic microorganisms (anoxiphotobacteria and cyanobacteria) with trace elements in aqueous solutions. Geochem Internat 45:302–307
Priester JH, Olson SG, Webb SM, Neu MP, Hersman LE, Holden PA (2006) Enhanced exopolymer production and chromium stabilization in Pseudomonas putida unsaturated biofilms. Appl Environ Microb 72:1988–1996
Raungsomboon S, Chidthaisong A, Bunnag B, Inthorn D, Harvey NW (2007) Lead (Pb2+) adsorption characteristics and sugar composition of capsular polysaccharides of cyanobacterium Calothrix marchica. Songklanakarin J Sci Technol 29(2):529–541
Reed RH, Richardson DL, Warr SRC, Stewart WDP (1984) Carbohydrate accumulation and osmotic stress in cyanobacteria. J Gen Microbiol 130:1–4
Rippka R, Deruelles J, Waterbury JB, Herdman MR, Stanier Y (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Selbmann L, Onofri S, Fenice M, Federici F, Petruccioli M (2002) Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Res Microbiol 153:585–592
Shah V, Ray A, Garg N, Madamwar D (2000) Characterization of the extracellular polysaccharide produced by a marine cyanobacterium, Cyanothece sp. ATCC 51142, and its exploitation toward metal removal from solutions. Curr Microbiol 40:274–278
Sheng GP, Yu HQ, Yue Z (2006) Factors influencing the production of extracellular polymeric substances by Rhodopseudomonas acidophila. Int Biodeter Biodegr 58:89–93
Shu CH, Hsu HJ (2008) Effects of sodium chloride on the production of bioactive exopolysaccharides in submerged cultures of Phellinus linteus. J Chem Technol Biotechnol 83:618–624
Sutherland IW (2001) Microbial polysaccharides from Gram-negative bacteria. Int Dairy J 11:663–674
Taraldsvik M, Myklestad SM (2000) The effect of pH on growth rate, biochemical composition and extracellular carbohydrate production of the marine diatom Skeletonema costatum. J Phycol 36:189–194
Thacker RW, Starnes S (2003) Host specificity of symbiotic cyanobacteria, Oscillatoria spongeliae, in marine sponges, Dysidea spp. Mar Biol 142:643–648
Torino MI, Taranto MP, Sesma F, de Valdez GF (2001) Heterofermentative pattern and exopolysaccharide production by Lactobacillus helveticus ATCC 15807 in response to environmental pH. J Appl Microbiol 91:846–852
Vanhaverbeke C, Heyraud A, Mazeau K (2003) Conformational analysis of the exopolysaccharide from Burkholderia caribensis strain MWAP71: impact of the interaction with soils. Biopolymers 69:480–497
Yee N, Benning LG, Phoenix VR, Ferris FG (2004) Characterization of metal-cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environ Sci Technol 38:775–782
Zou X, Sun M, Guo X (2006) Quantitative response of cell growth and polysaccharide biosynthesis by the medicinal mushroom Phellinus linteus to NaCl in the medium. World J Microb Biotechnol 22:1129–1133
Acknowledgements
Prof. Dr. Soner Gonen is gratefully acknowledged for his contribution to the statistical analysis. This research was supported by the Gazi University B.A.P. (05/2005-31).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozturk, S., Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ Sci Pollut Res 17, 595–602 (2010). https://doi.org/10.1007/s11356-009-0233-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0233-2