Abstract
Background, aims, and scope
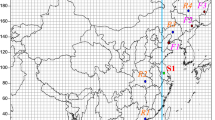
Since toxaphene (polychlorocamphene, polychloropinene, or strobane) mixtures were applied for massive insecticide use in the 1960s to replace the use of DDT, some of their congeners have been found at high latitudes far away from the usage areas. Especially polychlorinated bornanes have demonstrated dominating congeners transported by air up to the Arctic areas. Environmental fate modeling has been applied to monitor this phenomenon using parallel zones of atmosphere around the globe as interconnected environments. These zones, shown in many meteorological maps, however, may not be the best way to configure atmospheric transport in air trajectories. The latter could also be covered by connecting a chain of simple model boxes. We aim to study this alternative approach by modeling the trajectory chain using catchment boxes of our FATEMOD model. Polychlorobornanes analyzed in biota of the Barents Sea offered one case to study this modeling alternative, while toxaphene has been and partly still is used massively at southern East Europe and around rivers flowing to the Aral Sea.
Materials and methods
Pure model substances of three polychlorobornanes (toxaphene congeners P26, P50, and P62) were synthesized, their environmentally important thermal properties measured by differential scanning calorimetry, as evaluated from literature data, and their temperature dependences estimated by the QSPR programs VPLEST, WATSOLU, and TDLKOW. The evaluated property parameters were used to model their atmospheric long-range transport from toxaphene heavy usage areas in Ukraine and Aral/SyrDarja/AmuDarja region areas, through East Europe and Northern Norway (Finnmarken) to the Barents Sea. The time period used for the emission model was June 1997. Usual weather conditions in June were applied in the model, which was constructed by chaining FATEMOD model boxes of the catchment’s areas along assumed maximal air flow trajectories. Analysis of the three chlorobornanes in toxaphene mixtures function as a basis for the estimates of emission levels caused by its usage. High estimate (A) was taken from contents in a Western product chlorocamphene and low estimate (B) from mean contents in Russian polychloroterpene products to achieve modeled water concentrations. Bioaccumulation to analyzed lipid of aquatic biota at the target region was estimated by using statistical calculation for persistent organic pollutants in literature.
Results
The results from model runs A and B (high and low emission estimate) for levels in sea biota were compared to analysis results of samples taken in August 1997 at Barents Sea. The model results (ng g−1 lw): 4–95 in lipid of planktovores and 7–150 in lipid of piscivores, were in fair agreement with the analysis results from August 1997: 21–31 in Themisto libellula (chatka), 26–42 in Boreocadus saida (Polar cod), and 5–27 in Gadus morhua (cod) liver.
Discussion
The modeling results indicate that the application of chained simple multimedia catchment boxes on predicted trajectory is a useful method for estimation of volatile airborne persistent chemical exposures to biota in remote areas. For hazard assessment of these pollutants, their properties, especially temperature dependences, must be estimated by a reasonable accuracy. That can be achieved by using measurements in laboratory with pure model compounds and estimation of properties by thermodynamic QSPR methods. The property parameters can be validated by comparing their values at an environmental temperature range with measured or QSPR-estimated values derived by independent methods. The chained box method used for long-range air transport modeling can be more suitable than global parallel zones modeling used earlier, provided that the main airflow trajectories and properties of transported pollutants are predictable enough.
Conclusions
Long-range air transport modeling of persistent, especially photo-resistant organic compounds using a chain of joint simple boxes of catchment’s environments is a feasible method to predict concentrations of pollutants at the target area. This is justified from model results compared with analytical measurements in Barents Sea biota in August 1997: three of six modeled values were high and the other three low compared to the analysis results. The order of magnitude level was similar in both modeled (planktovore and piscivore) and observed (chatka and polar cod) values of lipid samples. The obtained results were too limited to firm validation but are sufficient to justify feasibility of the method, which prompts one to perform more studies on this modeling system.
Recommendations and perspectives
For assessment of the risk of environmental damages, chemical fate determination is an essential tool for chemical control, e.g., for EU following the REACH rules. The present conclusion of applicability of the chained single-box multimedia modeling can be validated by further studies using analyses of emissions and target biota in various other cases. To achieve useful results, fate models built with databases having automatic steps for most calculations and outputs accessible to all chemical control professionals are essential. Our FATEMOD program catchments at environments and compound properties listed in the database represent a feasible tool for local, regional, and, according our present test results, for global exposure predictions. As an extended use of model, emission estimates can be achieved by reversed modeling from analysis results of samples corresponding to the target area.



Similar content being viewed by others
References
AMAP (2002) Persistent organic pollutants in the Arctic. Monitoring and Assessment Programme. Oslo, 309 pp
Andrews P, Vetter W (1995) A systematic nomenclature system for toxaphene congeners Part 1: chlorinated bornanes. Chemosphere 31(8):3879–3886
Barrie L, Bidleman T, Dougherty D, Fellin P, Grift N, Muir D, Rosenberg B, Stern G, Toom D (1993) Atmospheric toxaphene in the high Arctic. Chemosphere 27(10):2037–2046
Bidleman TF, Leone AD (2004) Soil–air exchange of organochlorine pesticides in the Southern United States. Environ Pollut 128:49–57
Bidleman TF, Olney CE (1975) Long range transport of toxaphene insecticide in the atmosphere of the west North Atlantic. Nature (London UK) 257(5526):475–477
Bidleman TF, Wideqvist U, Jansson B, Söderlund R (1987) Organochlorine pesticides and polychlorinated biphenyls in the atmosphere of southern Sweden. Atmos Environ 21:641–654
Bidleman TF, Leone AD, Falconer RI (2003) Vapor pressures and enthalpies of vaporization for toxaphene congeners. J Chem Eng News 48:1122–1127
Burhenne J, Haintzl D, Xu L, Vieth B, Alder L, Parlar H (1993) Preparation and structure of high-chlorinated bornane derivatives for the quantification of toxaphene residues in environmental samples. Fresenius J Anal Chem 346:779–785
Chandurkar PS, Matsumura F (1979) Metabolism of toxicant B and toxicant C of toxaphene in rats. Bull Envir Contamin Toxicol 21(1):539–547
Chandurkar PS, Matsumura F, Ikeda T (1978) Identification and toxicity of toxicant AC, a toxic component of toxaphene. Chemosphere 7:123–130
Coelhan M, Maurer M (2005) Synthesis of two major toxaphene components and their photostabilities. J Agric Food Chem 58:10105–10112
El-Sebae AH, Abou Seid M, Saleh MA (1993) Status and environmental impact of toxaphene in the Third World—a case study of African agriculture. Chemosphere 27(10):2063–2072
Fedorov LA (1999) Persistent organic chemicals in the former Soviet Union. Environ Pollut 105:283–287
Foreid S, Rundberget T, Severinsen T, Wiig Ö, Skaare JU (2000) Determination of toxaphenes in fish and marine mammals. Chemosphere 41:521–528
Frenzen G, Hainzl D, Burhenne J, Parlar H (1994) Structures elucidation on the three most important toxaphene congeners by x-ray analyses. Chemosphere 28:2067–2074
Grain CF (1990) Tables 14.4 and 14.5, Eqn 14–25 (Chapter 14): vapor pressure. In: Lyman WJ, Reehl WF, Rosenblatt DH (eds) Handbook of chemical property estimation methods. ACS, Washington, DC
Harner T, Bidleman TF, Jantunen LMM, Mackay D (2001) Soil–air exchange model of persistent pesticides in the United States cotton belt. Environ Toxicol Chem 20(7):1612–1621
Heimstad ES, Smalås AO, Kallenborn R (2001) Environmental fate of chlorinated bornanes estimated by theoretical descriptors. Chemosphere 4:665–674
Jantunen LM, Bidleman TF (2003) Air–water gas exchange of toxaphene in Lake Superior. Envir Toxicol Chem 22(6):1229–1237
Jantunen LMM, Bidleman TF, Harner T, Parkhurst WJ (2000) Toxaphene, chlordane and other organochlorine pesticides in Alabama air. Environ Sci Technol 34:5097–5015
Kallenborn R, Oehme M, Vetter W, Parlar H (1994) Enantiomer selective separation of toxaphene congeners isolated from seal blubber and obtained by synthesis. Chemosphere 28(1):89–98
Karlsson H, Oehme M, Klungsöyr J (1997) 4,5-Dichloro-chlordene, a new synthetic internal standard for the quantification of toxaphene and chlordane congeners in fish from the Barents Sea and North Atlantic. Application and experience. Chemosphere 34(5–7):951–963
Lahtinen M, Paasivirta J, Nikiforov VA (2006) Evaluation of entropies of fusion of polychlorinated naphthalenes by model congeners: a DSC study. Thermochimica Acta 447:5–12
Lammel G, Klöppfer W, Semeena WS, Schmidt E, Leip A (2007) Multicompartmental fate of persistent substances. Comparison of predictions from multimedia box-models and a multicompartment chemistry-atmospheric transport model. Environ Sci Pollut Res 14(3):153–165
Li YF, Bidleman TF, Barrie LA (2001) Toxaphene in the United States 2. Emissions and residues. J Geophys Res [Atmospheres] 106(D16):17929–17938
Mackay D (1991) Multimedia Environmental Models. The Fugacity Approach. L242, Lewis, Chelsea, MI
MacLeod M, Woodfine D, Brimacombe J, Toose L, Mackay D (2002) A dynamic mass budget for Toxaphene in North America. Envir Toxicol Chem 21(8):1628–1637
Matsumura F, Howard RH, Nelson JO (1975) Structure of the toxic fraction A of toxaphene. Chemosphere 5:271–276
McConnell LL, Kucklick JR, Bidleman TF, Walla MD (1993) Long-range atmospheric transport of toxaphene to Lake Baikal. Chemosphere 27(10):2027–2036
Murphy TJ, Mullin MD, Meyer JA (1987) Equilibration of polychlorinated biphenyls and toxaphene with air and water. Environ Sci Technol 21:155–162
Nicholson HP (1968) Water contamination by pesticides. J Southeastern Section, Am Water Works Assoc 32(1):8–20
Nicholson HP, Grzenda AR, Teasley JI (1968) Water pollution by insecticides. J Southeastern Section, Am Water Works Assoc 32(1):21–27
Nikiforov VA, Karavan VA, Miltsov SA (1999) Analysis of camphechlor. Organohalogen Compounds 41:601–604
Nikiforov VA, Karavan VA, Miltsov SA (2000a) Relative retention times of chlorinated monoterpenes. Chemosphere 41(4):467–472
Nikiforov VA, Karavan VA, Miltsov SA (2000b) Relative retention times of 122 polychloroterpenes. Organohalogen Compounds 50:268–271
Nikiforov V, Trukhin A, Kruchkov F, Kiprianova A, Miltsov S, Kallenborg R (2004) Isolation of pure enantiomers of toxaphene congeners via hydrochlorination and chlorination of pinene and composition of Soviet polychloropinene. Organohalogen Compounds 66:456–461
Norstrom RJ, Simon M, Muir DCG, Schweinburg RE (1988) Organochlorine contaminants in Arctic marine food chains: identification, geographical distribution and temporal trends in Polar bears. Environ Sci Technol 22:1063–1071
Oehme M, Svendsen JS, Roland K, Klungsöyr J (1995) Characterization and application of synthetic 4,5-dichlorochlordene as internal standard for the quantification of toxaphene and chlordane congeners in fish from the Arctic. Organohalogen Compounds 26:333–338
Paasivirta J (2006) Estimation of vapour pressure, solubility in water, Henry’s law function, and Log Kow as a function of temperature for prediction of environmental fate of chemicals. In: Kungolos A, Brebbia AA, Samaras CP, Popov V (eds) Environmental toxicology. WIT transactions on biomedicine and health. vol. 10. WIT, Southampton, pp 11–20
Paasivirta J, Rantio T (1991) Chloroterpenes and other organochlorines in Baltic, Finnish and Arctic wildlife. Chemosphere 22(1–2):47–55
Paasivirta J, Rantio T, Koistinen J, Vuorinen PJM (1993) Studies on toxaphene in environment. II. PCCs in Baltic and Arctic Sea and lake fish. Chemosphere 27(10):2011–2015
Paasivirta J, Sinkkonen S, Mikkelson P, Rantio T, Wania F (1999) Estimation of vapour pressures, solubilities and Henry’s law constants of selected persistent organic pollutants as functions of temperature. Chemosphere 39(5):811–832
Paasivirta J, Sinkkonen S, Rantalainen A-L, Broman D, Zebühr Y (2002) Temperature dependent properties of environmentally important synthetic musks. Environ Sci Pollut Res 9(6):345–356
Pacyna JM, Brorström-Lunden E, Runge E, Paasivirta J, Münch J, Fudala J, Calamari D, Broman D (1999) Environmental cycling of selected persistent organic pollutants (POPs) in the Baltic Region. ENV4-CT96-0214/EU. Executive Final Summary Report, Annex 1: Technical Report and CD-ROM including: 1) Technical Report, 2) The Emission Database, 3) The Popcycling Model and 4) The Environmental Database. NILU, Kjeller
Parlar H, Angerhöfer D, Coelhan M, Kimmel L (1995) HRGC and HRGC-ECNI determination of toxaphene residues in fish with a new 22 components standard. Organohalogen Compounds 26:357–362
Pyysalo H, Antervo K (1985) GC profiles of chlorinated terpenes (toxaphenes) in some Finnish environmental samples. Chemosphere 14(11–12):1723–1728
Rantio T, Paasivirta J, Lahtiperä M (1993) Studies on toxaphene in environment. I. Experiences on analytical behavior of PCCs. Studies including pulp mill wastes. Chemosphere 27(10):2003–2010
Ruelle P (2000) The n-octanol and n-hexane/water partition coefficient of environmentally relevant chemicals predicted from the mobile order and disorder (MOD) thermodynamics. Chemosphere 40:457–512
Ruelle P, Kesselring UW (1997) Aqueous solubility prediction of environmentally important chemicals from the mobile order thermodynamics. Chemosphere 34:275–298
Ruelle P, Rey-Mermeth C, Buchmann M, Ho N-T, Kesselring UW, Huyskens PL (1991) A new predictive equation for the solubility of drugs based on the thermodynamics of mobile disorder. Pharm Res 8:840–850
Saleh MA (1991) Toxaphene: chemistry, biochemistry, toxicity and environmental fate. Rev Environ Contam Toxicol 118:1–85
Schwarzenbach RP, Cscwend PM, Imboden DM (1993) Environmental organic chemistry. Wiley, New York, pp 80–81
Semeena WS, Lammel G (2003) Effect of various scenarios upon entry of DDT and γ-HCH into the global environment on their fate as predicted by a multicompartment chemistry-transport model. Fresenius Environ Bull 12:925–939
Sinkkonen S, Paasivirta J (2000) Degradation half-life times of PCDDs, PCDFs and PCBs for environmental fate modelling. Chemosphere 40:943–949
Sleeswijk AW (2006) GLOBOX—a spatially differentiated multimedia fate and exposure model. Incl. instructions for download of GLOBACK. Environ Sci Pollut Res 13(2):143–144
Soermo EG, Pettersvik Salmer M, Jenssen BM, Hop H, Bek K, Kovacs KM, Lydersen C, Falk-Petersen S, Gabrielsen GW, Lie E, Skaare JU (2006) Biomagnification of polybrominated diphenyl ether and hexachloromonocyclododecane flame retardants in the Polar Bear food chain in Svalbard, Norway. Envir Toxicol Chem 25(9):2502–2511
Stern GA, Muir DCG, Ford CA, Grift NP, Dewailly E, Bidleman TF, Walla ND (1992) Isolation and identification of two major recalcitrant toxaphene congeners in aquatic biota. Environ Sci Technol 26:1838–1840
Sundström G (1981) Toxafen in Svensk Miljö—Tillförsel via Luftnedfall (in Swedish), NORDFORSK miljövårdserien publikation 1981(1):331–336
Swackhamer DL, McConnell LL, Gregor DJ (1993) Workgroup report on environmental transport and fate. Chemosphere 27(10):1837–1840
Swackhamer DL, Schottler S, Pearson RF (1999) Air–water exchange and mass balance of toxaphene in the Great Lakes. Environ Sci Technol 33:3864–3872
Swanson KL, Hope H, Landrum PF (1978) A toxaphene component: 2,5,6-exo-,8,8,9,10-heptachloro-dihydrocamphene. Acta Cryst B34:3411–3414
Tabor EG (1966) Contamination of urban air through the use of insecticides. Trans NY Acad Sci 28(5):569–578
Tarhanen J, Koistinen J, Paasivirta J, Vuorinen PJ, Koivusaari J, Nuuja I, Kannan N, Tatsukawa R (1989) Toxic significance of planar aromatic compounds in Baltic ecosystem—new studies of extremely toxic coplanar PCBs. Chemosphere 18(1–6):1069–1077
Trapp S, Matthies M (1998) Chemodynamics and environmental modeling. An introduction. CemoS user’s manual and diskette. Springer, Berlin
Tribulovich VG, Nikiforov VA, Karavan SA, Bolshakov S (1994) Synthesis and characterization of toxaphene congeners. Organohalogen Compounds 19:97–101
Turner WV, Engel JL, Casida JE (1977) Toxaphene components and related compounds: preparation and toxicity of some hepta-, octa-, and nonachlorobornanes, hexa- and heptachlorobornenes, and a hexachlorobornadiene. J Agric Food Chem 25(6):1394–1401
Vaz R, Blomqvist G (1985) Traces of toxaphene components in Swedish breast milk analyzed by capillary GC using ECD, electron impact and negative ion chemical ionization MS. Chemosphere 14(2):223–231
Vetter W, Scherer G (1998) Molecular modelling: an interesting tool to explain persistence and lability of compounds of technical toxaphene. Organohalogen Compounds 35:235–238
Vetter W, Oehme M (2000) Toxaphene. Analysis and environmental fate of congeners. In: Paasivirta J (ed) The handbook of environmental chemistry. Vol 3 Part K New types of persistent halogenated compounds. Springer, Berlin, pp 237–287
Voldner EC, Li YF (1993) Global usage of toxaphene. Chemosphere 27(10):2073–2078
Voldner EC, Li YF (1995) Global usage of selected persistent organic chemicals. Sci Total Environ 160/161:201–210
Voutsas E, Magoulas K, Tassios D (2002) Prediction of the bioaccumulation of persistent organic pollutants in aquatic food webs. Chemosphere 48:645–651
Wania F, Mackay D (1993a) Global fractionation and cold condensation of low volatility organochlorine compounds in Polar regions. Ambio 22:10–18
Wania F, Mackay D (1993b) Modelling the global distribution of Toxaphene: a discussion of feasibility and desirability. Chemosphere 27(10):2079–2094
Wania F, Persson J, di Guardo A, McLahlan M (2000) The POPCYCLING-Baltic model—a non-steady state multicompartment mass balance model of the fate of persistent organic pollutants in the Baltic Sea environment. Norwegian Institute of Air Research Report No. NILU OR 10/2000, Kjeller Norway
Wolkers H, Burkov IC, Lydersen C, Witkamp RF (2000) Chlorinated pesticide concentrations, with an emphasis on polychlorinated camphenes (toxaphenes), in relation to cytochrome P450 enzyme activities in harp seals (Phoca groenlandia) from the Barents Sea. Environ Toxicol Chem 19(6):1632–1637
Zell M, Ballschmiter K (1980) Baseline studies of global pollution II. Global occurrence of hexachlorobenzene and polychlorocamphenes (Toxaphene*) (PCC) in biological samples. Fresenius Z Anal Chem 300:387–402
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cafer Turgut
This article is dedicated to the memory of Professor Alexander B Terentiev (who passed away in November 2006), our true friend. With his Institute of Organo-Element Compounds, Russian Academy of Science, Moscow, he was an important main organizer of the six joint Finnish–Russian seminars (every third year since 1989) on the field (‘Chemistry and Ecology of Organo-Element Compounds’). He prompted us especially to search properties and environmental fates for various polyhalogen compounds. We remember him for his friendly character and great sense of humor.
Rights and permissions
About this article
Cite this article
Paasivirta, J., Sinkkonen, S., Nikiforov, V. et al. Long-range atmospheric transport of three toxaphene congeners across Europe. Modeling by chained single-box FATEMOD program. Environ Sci Pollut Res 16, 191–205 (2009). https://doi.org/10.1007/s11356-008-0084-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-008-0084-2