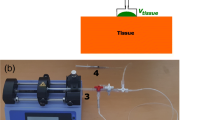
Abstract
Background
In vivo mechanical characterisation of biological soft tissue is challenging, even under moderate quasi-static loading. Clinical application of suction-based methods is hindered by usual assumptions of tissues homogeneity and/or time-consuming acquisitions/postprocessing.
Objective
Provide practical and unexpensive suction-based mechanical characterisation of soft tissues considered as bilayered structures. Inverse identification of the bilayers’ Young’s moduli should be performed in almost real-time.
Methods
An original suction system is proposed based on volume measurements. Cyclic partial vacuum is applied under small deformation using suction cups of aperture diameters ranging from 4 to 30 mm. An inverse methodology provides both bilayer elastic stiffnesses, and optionally the upper layer thickness, based on the interpolation of an off-line finite element database. The setup is validated on silicone bilayer phantoms, then tested in vivo on the abdomen skin of one healthy volunteer.
Results
On bilayer silicone phantoms, Young’s moduli identified by suction or uniaxial tension presented a relative difference lower than 10 % (upper layer thickness of 3 mm). Preliminary tests on in vivo abdomen tissue provided skin and underlying adipose tissue Young’s Moduli at 54 kPa and 4.8 kPa respectively. Inverse identification process was performed in less than one minute.
Conclusions
This approach is promising to evaluate elastic moduli in vivo at small strain of bilayered tissues.












Similar content being viewed by others
Notes
lsqnonlin function in MATLAB
Two main components of Skin FX10 110019
Deadner Skin FX10 110020
3D printer Prusa MK3S+
Aixplorer, probe SuperLinear™ SLH20-6
Aixplorer, probe SuperLinear™ SL10-2
References
Payan Y (ed) (2012) Soft Tissue Biomechanical Modeling for Computer Assisted Surgery. Springer, Berlin
Budday S, Sommer G, Birkl C, Langkammer C, Haybaeck J, Kohnert J, Bauer M, Paulsen F, Steinmann P, Kuhl E et al (2017) Mechanical characterization of human brain tissue. Acta Biomater 48:319–340
Gao Z, Lister K, Desai JP (2010) Constitutive modeling of liver tissue: experiment and theory. Ann Biomed Eng 38(2):505–516
Girard E, Chagnon G, Gremen E, Calvez M, Masri C, Boutonnat J, Trilling B, Nottelet B (2019) Biomechanical behaviour of human bile duct wall and impact of cadaveric preservation processes. J Mech Behav Biomed Mater 98:291–300
Kumaraswamy N, Khatam H, Reece GP, Fingeret MC, Markey MK, Ravi-Chandar K (2017) Mechanical response of human female breast skin under uniaxial stretching. J Mech Behav Biomed Mater 74:164–175
Masri C, Chagnon G, Favier D, Sartelet H, Girard E (2018) Experimental characterization and constitutive modeling of the biomechanical behavior of male human urethral tissues validated by histological observations. Biomech Model Mechanobiol 17(4):939–950
Avazmohammadi R, Li DS, Leahy T, Shih E, Soares JS, Gorman JH, Gorman RC, Sacks MS (2018) An integrated inverse model-experimental approach to determine soft tissue three-dimensional constitutive parameters: application to post-infarcted myocardium. Biomech Model Mechanobiol 17(1):31–53
Diab M, Kumaraswamy N, Reece GP, Hanson SE, Fingeret MC, Markey MK, Ravi-Chandar K (2020) Characterization of human female breast and abdominal skin elasticity using a bulge test. J Mech Behav Biomed Mater 103
Tonge TK, Atlan LS, Voo LM, Nguyen TD (2013) Full-field bulge test for planar anisotropic tissues: Part i-experimental methods applied to human skin tissue. Acta Biomater 9(4):5913–5925
Cox MA, Driessen NJ, Boerboom RA, Bouten CV, Baaijens FP (2008) Mechanical characterization of anisotropic planar biological soft tissues using finite indentation: experimental feasibility. J Biomech 41(2):422–429
Samani A, Zubovits J, Plewes D (2007) Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol 52(6):1565
Zhao R, Sider KL, Simmons CA (2011) Measurement of layer-specific mechanical properties in multilayered biomaterials by micropipette aspiration. Acta Biomater 7(3):1220–1227. https://doi.org/10.1016/j.actbio.2010.11.004
Gefen A, Margulies SS (2004) Are in vivo and in situ brain tissues mechanically similar? J Biomech 37(9):1339–1352
Kerdok AE, Ottensmeyer MP, Howe RD (2006) Effects of perfusion on the viscoelastic characteristics of liver. J Biomech 39(12):2221–2231
Ottensmeyer MP (2002) In vivo measurement of solid organ visco-elastic properties. Stud Health Technol Inform pp. 328–333
Elahi SA, Connesson N, Chagnon G, Payan Y (2019) In-vivo soft tissues mechanical characterization: Volume-based aspiration method validated on silicones. Exp Mech 59(2):251–261
Fougeron N, Rohan PY, Haering D, Rose JL, Bonnet X, Pillet H (2020) Combining freehand ultrasound-based indentation and inverse finite element modeling for the identification of hyperelastic material properties of thigh soft tissues. J Biomech Eng 142(9). https://doi.org/10.1115/1.4046444
Frauziols F, Chassagne F, Badel P, Navarro L, Molimard J, Curt N, Avril S (2016) In vivo identification of the passive mechanical properties of deep soft tissues in the human leg. Strain 52(5):400–411. https://doi.org/10.1111/str.12204
Ranger BJ, Moerman KM, Anthony BW, Herr HM (2023) Constitutive parameter identification of transtibial residual limb soft tissue using ultrasound indentation and shear wave elastography. J Mech Behav Biomed Mater 137:105541. https://doi.org/10.1016/j.jmbbm.2022.105541
Sengeh DM, Moerman KM, Petron A, Herr H (2016) Multi-material 3-d viscoelastic model of a transtibial residuum from in-vivo indentation and MRI data. J Mech Behav Biomed Mater 59:379–392. https://doi.org/10.1016/j.jmbbm.2016.02.020
Wei H, Liu X, Dai A, Li L, Li C, Wang S, Wang Z (2021) In vivo measurement of the mechanical properties of facial soft tissue using a bi-layer material model. Int J Appl Mech 13(03):2150034
Xu M, Yang J (2015) Human facial soft tissue thickness and mechanical properties: a literature review. In: International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. Am Soc Mech Eng 57045: V01AT02A045
Diridollou S, Berson M, Vabre V, Black D, Karlsson B, Auriol F, Gregoire J, Yvon C, Vaillant L, Gall Y, Patat F (1998) An in vivo method for measuring the mechanical properties of the skin using ultrasound. Ultrasound Med Biol 24(2):215–224
Diridollou S, Patat F, Gens F, Vaillant L, Black D, Lagarde J, Gall Y, Berson M (2000) In vivo model of the mechanical properties of the human skin under suction. Skin Res Technol 6(4):214–221
Hendriks F, Dv Brokken, Van Eemeren J, Oomens C, Baaijens F, Horsten J (2003) A numerical-experimental method to characterize the non-linear mechanical behaviour of human skin. Skin Res Technol 9(3):274–283. https://doi.org/10.1016/j.medengphy.2005.07.001
Hendriks F, Brokken D, Oomens C, Bader D, Baaijens F (2006) The relative contributions of different skin layers to the mechanical behavior of human skin in vivo using suction experiments. Med Eng Phys 28(3):259–266. https://doi.org/10.1016/j.medengphy.2005.07.001
Badir S, Bajka M, Mazza E (2013) A novel procedure for the mechanical characterization of the uterine cervix during pregnancy. J Mech Behav Biomed Mater 27:143–153
Müller B, Elrod J, Pensalfini M, Hopf R, Distler O, Schiestl C, Mazza E (2018) A novel ultra-light suction device for mechanical characterization of skin. PLoS ONE 13(8)
Hollenstein M, Bugnard G, Joos R, Kropf S, Villiger P, Mazza E (2013) Towards laparoscopic tissue aspiration. Med Image Anal 17(8):1037–1045
Kauer M, Vuskovic V, Dual J, Székely G, Bajka M (2002) Inverse finite element characterization of soft tissues. Med Image Anal 6(3):275–287
Lakhani P, Dwivedi KK, Parashar A, Kumar N (2021) Non-invasive in vivo quantification of directional dependent variation in mechanical properties for human skin. Front Bioeng Biotechnol 9
Nava A, Mazza E, Furrer M, Villiger P, Reinhart W (2008) In vivo mechanical characterization of human liver. Med Image Anal 12(2):203–216
Röhrnbauer B, Betschart C, Perucchini D, Bajka M, Fink D, Maake C, Mazza E, Scheiner DA (2017) Measuring tissue displacement of the anterior vaginal wall using the novel aspiration technique in vivo. Sci Rep 7(1):1–7
Schiavone P, Chassat F, Boudou T, Promayon E, Valdivia F, Payan Y (2009) In vivo measurement of human brain elasticity using a light aspiration device. Med Image Anal 13(4):673–678
Vuskovic V (2001) Device for in-vivo measurement of mechanical properties of internal human soft tissues. PhD thesis, ETH Zurich
Weickenmeier J, Jabareen M, Mazza E (2015) Suction based mechanical characterization of superficial facial soft tissues. J Biomech 48(16):4279–4286
Luboz V, Promayon E, Payan Y (2014) Linear elastic properties of the facial soft tissues using an aspiration device: towards patient specific characterization. Ann Biomed Eng 42(11):2369–2378
Elahi SA, Connesson N, Payan Y (2018) Disposable system for in-vivo mechanical characterization of soft tissues based on volume measurement. J Mech Med Biol 18(04):1850037. https://doi.org/10.1142/S0219519418500379
Kappert K, Connesson N, Elahi S, Boonstra S, Balm A, van der Heijden F, Payan Y (2021) In-vivo tongue stiffness measured by aspiration: Resting vs general anesthesia. J Biomech 114
Barbarino GG, Jabareen M, Mazza E (2011) Experimental and numerical study on the mechanical behavior of the superficial layers of the face. Skin Res Technol 17(4):434–444. https://doi.org/10.1111/j.1600-0846.2011.00515.x
Sachs D, Wahlsten A, Kozerke S, Restivo G, Mazza E (2021) A biphasic multilayer computational model of human skin. Biomech Model Mechanobiol 20(3):969–982
MacKinnon JG (2013) Thirty years of heteroskedasticity-robust inference. In: Recent advances and future directions in causality, prediction, and specification analysis, Springer, pp 437–461
Sidik K, Jonkman JN (2016) A comparison of the variance estimation methods for heteroscedastic nonlinear models. Stat Med 35(26):4856–4874. https://doi.org/10.1002/sim.7024
Douglas MBates DGW (1988) Nonlinear Regression Analysis and Its Applications. John Wiley & Sons, Inc. https://doi.org/10.1002/9780470316757
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11(2):431–441
Shao J (1990) Asymptotic theory in heteroscedastic nonlinear models. Statist Probab Lett 10(1):77–85
Jemec G, Jemec B, Jemec B, Serup J (1990) The effect of superficial hydration on the mechanical properties of human skin in vivo: implications for plastic surgery. Plast Reconstr Surg 85(1):100–103
Park A (1972) Rheology of stratum corneum-i: A molecular interpretation of the stress-strain curve. J Soc Cosmet Chem 23:3–12
Joodaki H, Panzer MB (2018) Skin mechanical properties and modeling: A review. Proc Inst Mech Eng Part H: J Eng Med 232(4):323–343
Kalra A, Lowe A, Al-Jumaily A (2016) Mechanical behaviour of skin: a review. J Mater Sci Eng 5(4):1000254
Mostafavi Yazdi SJ, Baqersad J (2022) Mechanical modeling and characterization of human skin: A Rev J Biomech 130:110864
Mîra A, Carton AK, Muller S, Payan Y (2018) A biomechanical breast model evaluated with respect to mri data collected in three different positions. Clin Biomech Elsevier Ltd 60:191–199
Nazari MA, Perrier P, Chabanas M, Payan Y (2010) Simulation of dynamic orofacial movements using a constitutive law varying with muscle activation. Comput Methods Biomech Biomed Engin 13(4):469–482
Pensalfini M, Weickenmeier J, Rominger M, Santoprete R, Distler O, Mazza E (2018) Location-specific mechanical response and morphology of facial soft tissues. J Mech Behav Biomed Mater 78:108–115
Mukhina E, Rohan PY, Connesson N, Payan Y (2020) Calibration of the fat and muscle hyperelastic material parameters for the assessment of the internal tissue deformation in relation to pressure ulcer prevention. Comput Methods Biomech Biomed Engin 23(sup1):S197–S199. https://doi.org/10.1080/10255842.2020.1813426
Bucki M, Luboz V, Perrier A, Champion E, Diot B, Vuillerme N, Payan Y (2016) Clinical workflow for personalized foot pressure ulcer prevention. Med Eng Phys 38(9):845–853
Chinesta F, Cueto E (2015) Techniques de reduction de modeles vers une nouvelle generation d’abaques numeriques. Techniques de l’ingenieur Mathematiques base documentaire : TIP052WEB.(ref. article : af1381), 10.51257/a-v1-af1381, https://www.techniques-ingenieur.fr/base-documentaire/sciences-fondamentales-th8/analyse-numerique-des-equations-differentielles-et-aux-derivees-partielles-42620210/techniques-de-reduction-de-modeles-af1381/, fre,base documentaire : TIP052WEB
Aoki T, Ohashi T, Matsumoto T, Sato M (1997) The pipette aspiration applied to the local stiffness measurement of soft tissues. Ann Biomed Eng 25(3):581–587
Aversano G, Bellemans A, Li Z, Coussement A, Gicquel O, Parente A (2019) Application of reduced-order models based on pca & kriging for the development of digital twins of reacting flow applications. Computers & Chemical Engineering 121:422–441
Horn SD, Horn RA, Duncan DB (1975) Estimating heteroscedastic variances in linear models. Journal of the American Statistical Association 70(350):380–385, http://www.jstor.org/stable/2285827
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this article.
Appendices
Appendix A: Real time Evaluation of the Simulated Apparent Stiffness
The apparent stiffness \(B_{i\, SIM}(\beta , \theta )\) is the slope of the pressure-shape curve at shape \(S=0.1\) (equation (6), main paper body) when aspirating a bilayer phantom. This simulated stiffness is evaluated many times to find iteratively the minimum of the cost function \(\Phi _{Param}\) (equation (8), main paper body) or to evaluate the identifiability of the material parameters (Appendix B). The apparent stiffness \(B_{i\, SIM}(\beta , \theta )\) depends mainly on the different combination of four parameters, which are the aperture diameter \(D_i\), the upper layer Young’s modulus \(E_{R1}\) and its thickness \(L_{R1}\), and the lower layer Young’s modulus \(E_{R0}\) (Fig. 3).
If the simulated apparent stiffness \(B_{i\, SIM}(\beta , \theta )\) was evaluated using, for example, a FE model implemented and updated for each calculation point, the time required to solve a single inverse identification would be phenomenal. Therefore, this appendix describes how the simulated apparent stiffness \(B_{i\, SIM}(\beta , \theta )\) was evaluated in real time. The idea is mainly to define and interpolate precalculated abacuses as discussed in [57].
Four main steps are required:
-
1.
Reducing, if possible, the number of parameters required for the database ("Database Definition"),
-
2.
Defining a FE model for the suction experiment and creating the database in the required parameter range ("FE Model"),
-
3.
Interpolate the database for any parameters \(D_i\), \(L_{R_1}\), \(E_{R1}\) and \(E_{R0}\) ("Database Interpolation"),
-
4.
Validate the proposed method ("Validation").
A.1 Database definition
The four main parameters \(D_i\), \(L_{R_1}\), \(E_{R1}\) and \(E_{R0}\) can be combined to reduce the required dimension of the FE database from 4 to 2.
Scale Effect :
assuming the lower layer thickness is infinite (in practice, the total thickness of the layer is much larger than the aperture diameter \(D_i\)), the upper layer relative contribution to the shape \(S_{tissue}\) is governed only by the depth ratio \(\zeta =\frac{D_i}{L_{R_1}}\) between the aperture diameter \(D_i\) and upper layer thickness \(L_{R_1}\) [12]; redundant depth ratio \(\zeta\) provides redundant information in the FE database.
Material Stiffness Contrast :
considering a material stiffness contrast ratio \(\eta =\frac{E_{R1}}{E_{R0}}\), the apparent stiffness \(B_{i\, SIM}(\beta , \theta )\) can be seen as proportional to the bottom layer stiffness \(E_{R0}\) (equation (10)).
The required FE database to compute the apparent stiffness \(B_{i\, SIM}(\beta , \theta )\) can thus be reduced to evaluate a two-parameter function, \(f_{sim}\), so that:
where \(f_{sim}\) is an adimensional function depending on the depth ratio \(\zeta =\frac{D_i}{L_{R_1}}\) and on the layer stiffness contrast ratio \(\eta =\frac{E_{R1}}{E_{R0}}\).
Note that equation (10) implies that the cost function \(\Phi _{Param}\) (equation (8), main paper body) is linearly conditional on the parameter \(E_{R0}\) [44]. It means that once \(\zeta\) and \(\eta\) are chosen, the parameter \(E_{R0}\) minimising \(\Phi _{Param}\) is simply obtained by solving a linear problem.
The range of both the ratio parameters \(\zeta\) and \(\eta\) were chosen to build the database, i.e. to estimate the function \(f_{sim}(\zeta , \eta )\) :
-
1.
The chosen range for the stiffness contrast ratio \(\eta\) was from 1 to 120 to anticipate application to in-vivo cases.
-
2.
Aspirating with an aperture of diameter \(D_i\) extracts data mainly at a depth of one diameter [12]. Let us consider the case where the layer thickness is greater than \(D_i\), i.e. for example, \(L_{R_1}>3 D_i\). A small increase of the layer thickness should have negligible influence on the result in this case [12, 36, 40]. Therefore, a limit scale ratio \(\zeta =\frac{D_i}{L_{R_1}}>\frac{1}{3}\) was chosen. Moreover, the smallest aperture diameter being of \(D_{i}=4\) mm, it was decided that the identification of mechanical properties of layers thinner than 0.25 mm would be out of the identification range of this work. The largest aperture diameter being of \(D_{i}=30\) mm, the maximum depth ratio \(\zeta\) for such a thin layer is of 120. Therefore, the range of the scale ratio \(\zeta\) required in this work was \([\frac{1}{3}, \, 120]\).
FE Model
Model Definition
An FE model was parameterized using a Matlab code to provide \(f_{sim}(\zeta , \eta )\) for the chosen ranges of \(\zeta\) and \(\eta\):
where \(B_{i\, SIM\, db}\) is the slope of the FE pressure-shape curve. To compute the database, an arbitrarily chosen lower layer stiffness of \(E_{R0\, db}=4000\) Pa was used.
A static, implicit, axisymmetric model (ANSYS APDL) was defined to describe suction onto cylindrical phantoms. The model takes into account large displacements. A constant aperture diameter of \(D_{i}=10\) mm was chosen (Fig. 13(a)); the depth ratio \(\zeta =\frac{D_i}{L_{R_1}}\) was changed by modifying the layer thickness \(L_{R_1}\). To allow the use of a unique mesh for all simulations in the database, a geometry of \(M=20\) pre-meshed layers was defined (Fig. 13(b)). The ratio \(\zeta =\frac{D_i}{L_{R_1}}\) was thus modified between simulations by attributing a Young modulus of \(E_{R1\, db}\) to the first \([1, \, m]\) upper-layers and a Young’s modulus of \(E_{R0\, db}\) to the other layers in \([m+1, \, M]\). The mesh used to compute the whole database was composed of 6 bilinear axisymmetrical elements (Q8, Plane183, ANSYS) in each layer thickness. A zoom-in of the mesh size is reported in Fig. 13(b).
Note that the parts of the 3D printed cups in contact with the tissue (wall thickness, fillet radius) were all proportional to the cup aperture \(D_i\); the model cup geometry in contact with the phantom is representative of the reality for all cup sizes.
The boundary conditions are presented in Fig. 13(a). The vertical line AG is the axisymetric axis of the model; a single planar section of the model defines the whole model geometry. The top of the suction aperture (line CD) is clamped in all directions. A partial vacuum \(-\Delta P_{tissue}\) is applied to the line AB. Contact elements were defined between the suction aperture and the line AB. With these boundary conditions, the whole tissue is free to move up or down relatively to the cup, depending on the applied pressure \(-\Delta P_{tissue}\). These boundary conditions account for the fact that external loads applied on the cup were as small as possible during the experiments (Fig. 4, illustration on phantom A). No additional external loads were taken into account in the simulations. Furthermore, the dimensions of the phantom were large enough so that the application of a rigid casing outside the tissue phantom (Fig. 13(a)) had a negligible impact on the aspirated volume (numerically tested).
The material of aperture and, optionally of the rigid casing, were modelled with an elastic Hookean model with steel mechanical properties. An incompressible Neo-Hookean model simulated the material behaviour of each tissue layer. The apparent stiffness \(B_{i\, SIM\, db}\) was evaluated at shapes equal to 0.1; for such a small deformation state, the incompressibility of the material (Poisson coefficient \(\nu \in [0.45\, \, 0.5[\)) did not influence the results (numerically tested).
The friction coefficient between the tissue and the cup was chosen of \(f=0.2\). During the experiment, this parameter was actually unknown and was affected by the ultrasound gel cord. The influence of the friction coefficient has been tested numerically (no friction to glued boundary conditions). Its effect was considered negligible (as also reported in [58]) when the upper layer is stiffer than the lower layer.
The model solution was computed for an initial small partial vacuum \(-\Delta P_{tissue}\). The 2D displacement of line AB was converted by numerical integration into the simulated volume \(V_{tissue}\) aspirated into the cup. This volume was normalised into shape \(S_{tissue}\) (equation (4)), main paper body). The partial vacuum \(-\Delta P_{tissue}\) was gradually and monotonically increased. The output result needed to include the shape \(S_{tissue}=0.1\) to be validated (Fig. 14(a)). The results obtained around this reference shape were used to compute the sought slope \(B_{i\, SIM db}\), which provided in turn the adimensional value \(f_{sim}\) (equation (11), illustration in Fig. 14(b)) for \(\eta =\frac{E_{R1}}{E_{R0}}=120\)).
The FE database was calculated on stiffness ratios ranges: \(\eta =\frac{E_{R1}}{E_{R0}}\in [1, \, 120]\) and \(\zeta =\frac{D_i}{L_{R_1}} \in [\frac{1}{3},~133]\) (Fig. 15).
Mesh Convergence
To be trustworthy, the database results should be independent of the mesh used. To test this point, a specific curve of the simulation output \(f_{sim}\) is presented for 6 meshes with different sizes. Mesh 1 is the coarsest mesh, with only 1 elements in each pre-meshed layer thickness (\(6\,561\) elements in the tissue). The number of elements in each pre-meshed layer thickness is progressively increased up to 6 elements (\(65\,918\) elements in the tissue). The thinnest mesh is noted Mesh 6 (Fig. 13(b)).
The case with the stiffness contrast ratio \(\eta =\frac{E_{R1}}{E_{R0}}=120\) was considered to be the most demanding case, i.e. inducing stress concentrations that could most affect the results. The curve of interest \(f_{sim}\) is presented in (Fig. 14(b)) for all 6 meshes. At first sight, all the results overlap. A closer inspection (zoom-in Fig. 14(b)) confirms that the curves obtained for all 6 meshes are slightly different. The convergence of this curve is illustrated in Fig. 14(c) for different depth ratios \(\zeta =\frac{D_i}{L_{R_1}}\) and taking the output curve of Mesh 6 as reference to compute relative variations. Therefore, the variations between Mesh 1 and 6 are less than \(2\%\) even if the total number of elements is multiplied by 10. Mesh 6 is considered converged and has been used to compute the entire database.
Database Interpolation
The database (Fig. 15) was analysed using the Principal Component Analysis (PCA) method (based on the well known Singular Value Decomposition method). For a detailed description of the model reduction using the PCA method, the reader is kindly referred to [59]. Only the 3 first eigenvectors and associated weighting functions were kept, representing more than \(99.99\%\) of the database information:
where \(V_p(\zeta )\) are the three first PCA normalised eigen vectors and \(\alpha _p(\eta )\) are the associated weighing functions. \(f_{sim0}=0.7885\) is the FE output for a stiffness ratio \(\eta =1\) subtracted from the database prior to PCA. The eigen vectors \(V_p(\zeta )\) and their spline interpolation are presented in Fig. 16(a). The weighing functions \(\alpha _p(\eta )\) are presented in Fig. 16(b) and (c). Note that the database is dominated by the first weighing function \(\alpha _1(\eta )\) and associated first eigen vector \(V_1(\zeta )\); the simulated value \(f_{sim\, PCA}\) is mainly proportional to the first eigen vector \(V_1(\zeta )\).
Database interpolation results using the PCA is presented as black continuous curves in Fig. 15. Although each point of Fig. 15 required to solve a FE model for different partial vacuums \(-\Delta P_{tissue}\), the interpolation of the whole database requires only the interpolation of the eigen vectors and weighing function in equation (12). Also note that any other interpolation scheme could have been chosen to interpolate the FE database.
Validation
To validate the apparent stiffness \(B_{i\, SIM}(\beta , \theta )\) predicted by the PCA interpolation (equations (12) and (10)), additional tests were performed. Seven FE models were created with overmeshed models (200 elements in diameter \(D_i\)) and implementing the exact parameters \(D_i\), \(L_{R_1}\), \(E_{R1}\) and \(E_{R0}\). The other parameters of the model were kept similar to the ones used to compute the whole database.
The input parameters and the associated apparent stiffness results by direct FE simulation or PCA interpolation (\(B_{i\, SIM\, FE}\) and \(B_{i\, SIM\, PCA}\), respectively) are reported in Table 8. Note that both the dimension ratio \(\zeta\) and the stiffness ratio \(\eta\) were chosen so as not to be directly represented in the database (Figs. 15 and 16). For all tests performed, the relative error between the PCA and the direct FE model is less than \(1\%\), which is considered to be fully satisfactory.
Axisymmetric FE model. Subplot (a) Geometry, boundary conditions, and main dimensions. The nodes of the CD line are completely clamped. Line AG nodes cannot move horizontally and are free in the vertical direction to account for the axisymmetric conditions. A partial vacuum homogeneous pressure \(-\Delta P_{tissue}\) is applied to the AB line and is represented by the green area and arrows. Contact elements are defined between the line AB and the suction aperture. Note that with the defined boundaries conditions, the suction cup is fixed and the tissue can freely move up and down into the suction aperture under partial vacuum \(-\Delta P_{tissue}\). This set of boundary conditions ensures that load between tissue and suction aperture is only due to the cup internal pressure; no external normal or shear loads are added to the model. Subplot (b) Local mesh zoom in: pre-meshed layers are defined at different depths (\(L_{R_1}=\{0.075, \, 0.3, \, 0.67, \, 1.2, \, 1.8, \, ...\}\)) to use the same converged mesh for all calculations in the database (six Q8 element minimum in each layer thickness, noted Mesh 6). The mechanical property of the material \(E_1\) is applied to the elements of the upper pre-meshed layers (illustration of the layer thickness \(L_{R_1}=1.2\) presented as a darker gray, i.e. a ratio \(\zeta =8.3, m=4\))
Mesh convergence demonstration for a specific database curve \(f_{sim}\). Subplot (a) Pressure-shape curves obtained for the thinnest mesh (Mesh 6), for a stiffness ratio \(\eta =\frac{E_{R1}}{E_{R0}}=120\) and different values of the depth ratio \(\zeta =\frac{D_i}{L_{R_1}}\). Subplot (b) Simulation output curve \(f_{sim}\) for a stiffness ratio \(\eta =\frac{E_{R1}}{E_{R0}}=120\). The output curves for 6 different meshes (from coarse to thin) overlap in this plot. Local zoom-in for a depth ratio \(\zeta =\frac{D_i}{L_{R_1}}=3.7\) illustrates convergence with mesh refinement. Subplot (c) Relative variations of \(f_{sim}\) for meshes 1 to 6 (total number of elements in the model multiplied by 10) using the results of Mesh 6 as reference
The FE normalized results \(f_{sim}\) (equation (11)) in the database are represented as coloured point markers versus depth ratio \(\zeta =\frac{D_i}{L_{R_1}}\). The stiffness ratios range is \(\eta =\frac{E_{R1}}{E_{R0}} \in [1, \, 120]\). Interpolation of the PCA eigen vectors and weighing functions enables interpolation of the database (equation (12)), as presented with the black curves joining the point markers. Integer values of depths ratio \(\frac{D_i}{L_{R_1}}\) are visually represented under the abscissa axis. Illustrations of particular interpolated points \(f_{sim\, PCA}\) (equation (12)) used to compute the values \(B_{i\, SIM\, PCA}\) in Table 8 are also reported as specific markers. Consult Table 8 for corresponding legend
PCA three first eigen vectors and weighing functions representing the FE database (equation (11)). Illustrations of particular interpolated points on the eigen and weighing functions to compute \(f_{sim\, PCA}\) (equation (12)) and \(B_{i\, SIM\, PCA}\) (equation (10)) in Table 8 are also reported in this figure as specific markers. Consult Table 8 for corresponding legend. Subplot (a) Three first normalised eigen vectors and associated interpolation with splines. Subplot (b) Pondering functions \(\alpha _1\) and spline interpolation. Subplot (c) Pondering functions \(\alpha _2\) and \(\alpha _3\) and spline interpolation
Appendix B: Parameters’ Identifiability and Experimental Variance
As mentioned in the main body of the paper, choosing weights \(w_i^2\) representative of the experimental variance \(\sigma _{i}^2\) is important if the parameter identifiability is directly inferred from the cost function \(\Phi _{Param}\) (equation (8), main paper body). This appendix develops the mathematical approach chosen to evaluate the parameter identifiability and the variance estimation derived from the residual vector \(u_{ij}\).
Parameters’ Identifiability
The parameter identifiability under heteroscedastic variance is usually computed using different variance-covariance estimators [42, 43]. In this work, a classic variance-covariance matrix \(\widehat{V}_{WLS}\) is used [43]:
where \(F\big (\widehat{\beta }\big )\) is the \(N_m\times P\) Jacobian matrix of the function \(w_i\, Ln\big (B_{i\, SIM}(\beta , \theta )\big )\) (equation (8), main paper body) evaluated at \(\beta =\widehat{\beta }\) . The variance-covariance matrix \(\widehat{V}_{WLS}\) is of dimension \(P\times P\) and is a linear approximation of the inverse of the Hessian matrix of \(\Phi _{Param}\). Its graphical representation is an hyperelipsoid of dimension P known as Indifference Regions (IR). In this work, IR with a confidence level of \(95\%\) will be plotted.
With this approximation, the Confidence Interval (CI) for parameter \(\widehat{\beta }\) is computed as [43]:
where \(\widehat{\beta }_p\) is the pth element of \(\widehat{\beta }\) and \(z_{\alpha /2}\) is the cumulative distribution of a normally centered distribution function for a confidence level \(\alpha\).
Note that the particular residual error vector \(e_{ij}=w_i u_{ij}\), which is the residual value for a specific noise copy \(\epsilon _{ij}\), is not taken into account to compute the variance-covariance matrix \(\widehat{V}_{WLS}\) (equation (13)). The variances and associated weights \(w_i\), taken into account while computing the Jacobian matrix F of \(w_i\, Ln\big (B_{i\, SIM}(\beta , \theta )\big )\), must be properly estimated so that the calculated CIs are meaningful.
Input Noise Variance Evaluation
In this work, the variances \(\sigma _i^2\) of the noise copies \(\epsilon _{ij}\) (equation (7), main paper body) for each aperture diameter \(D_i\) were evaluated in two different ways.
Given equation (7) (main paper body), the classic way is to compare the experimental values \(Ln\big (B_{ij\, EXP}\big )_k\) obtained on the phantom k, aperture diameter \(D_i\) and cycle j, with the averaged value \(\overline{Ln\big ({B}_{ij\, EXP}\big )}_k\) over the number of cycles \(J_{ki}\) measured on the phantom k and with aperture diameter \(D_i\), so that:
where K is the number of phantoms, and \(J_{ki}\) is the total number of cycles for the phantom k and aperture diameter \(D_i\). Thus, the parameter \(N_{ki}= \sum _{k=1}^{K} J_{ki}\) is the number of tests that one has at hand for aperture diameter \(D_i\).
The unbiased variance \(\sigma ^{2}_{i\, Classic}\) is an exact evaluation under the hypothesis that the model perfectly fits the data and that the random disturbance \(\epsilon _{ij}\) is of zero mean: in equation (15), the average value \(\overline{Ln\big ({B}_{ij\, EXP}\big )}_k\) plays the role of a model that ’perfectly’ fits the data.
In the cases where these hypotheses are not perfectly met, the classic variance underestimates the actual variance. Another variance estimation, also known as the Almost Unbiased Estimator (AUE), has been implemented based on [60]:
where \(u_{ij\, k}\) is the residual error vector obtained on phantom k, aperture diameter \(D_i\) and cycle j after fitting a model on all phantom k experimental data (one cost function \(\phi _{param}\) per phantom k, (equation (8), main paper body). The leverages \(\widehat{h_{ij}}_k\) are the diagonal values of the ’hat’ matrix \(H_k\) of dimensions \(J_{ki} \times J_{ki}\) defined for the kth non-linear model on the phantom k. The hat matrix \(H_k\) defined for non-linear models on phantom k writes [43]:
where \(F_k\big (\widehat{\beta }\big )\) is the \(J_{ki}\times P\) Jacobian matrix of \(w_i\, Ln\big (B_{i\, SIM\, k}(\beta , \theta )\big )\) evaluated at \(\beta =\widehat{\beta }\) on the phantom k.
In this contribution, the AUE variance was computed iteratively. The starting weights were chosen so that \(w_i^2=1\) to define the function \(\Phi _{Param}\) in equation (8), (main paper body). The residual error vector \(u_{ij\, k}\) minimizing \(\Phi _{Param}\) (equation (9), main paper body) was then computed and injected in equation (16) to provide a variance estimation \(\sigma ^{2}_{i\, AUE}\). This estimation has then been used to compute new weights (\(w_i^2=1/\sigma ^{2}_{i\, AUE}\)) and a new iteration was performed. Iterations were performed until the convergence of \(\sigma ^{2}_{i\,AUE}\) (few iterations in practice).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Connesson, N., Briot, N., Rohan, P.Y. et al. Bilayer Stiffness Identification of Soft Tissues by Suction. Exp Mech 63, 715–742 (2023). https://doi.org/10.1007/s11340-023-00946-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-023-00946-x