Abstract
Background
Load-relaxation under a constant state of deformation is a common characteristic of hydrated materials, including hydrogels and biological tissues. Overall, mechanical response in such materials is a strong function of underlying structure, which in hydrogels depends on whether the gel is formed through physical or chemical cross-linking. In order to use hydrogels in biomedical applications where their properties are matched to those of native tissues, it is critical to understand these underlying structure-properties relationships.
Objective
The objective of current work is to quantitatively characterize the load-relaxation behavior of physical and chemical gels and perform a comparative analysis with several biological tissues.
Methods
Microindentation-based load-relaxation experiments were performed on three physical (agar, alginate, and gelatin) gels and one chemical (polyacrylamide) gel with a range of experimental time frames.
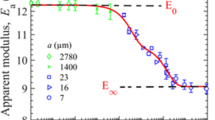
Results
All three physical gels exhibit strong time-dependent load-relaxation behavior where faster indentation leads to pronounced load-relaxation over short time-scales. The polyacrylamide gel is largely time-independent and exhibits negligible relaxation within short time-scales. The material property intrinsic permeability, which relates to underlying pore structure, was time-independent for both physical and chemical gels.
Conclusions
A comparative analysis reveals that different aspects of the time-dependent properties of biological tissues are captured by physical and chemical hydrogels, with implications for tissue engineering applications.










Similar content being viewed by others
References
Oyen ML (2014) Mechanical characterisation of hydrogel materials. Int Mater Rev 59(1):44–59
Spiller KL, Maher SA, Lowman AM (2011) Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev 17(4):281–299
Kumar PS, Raj NM, Praveen G, Chennazhi KP, Nair SV, Jayakumar R (2013) In vitro and in vivo evaluation of microporous chitosan hydrogel/nanofibrin composite bandage for skin tissue regeneration. Tissue Eng Part A 19(3–4):380–392
Ashley GW, Henise J, Reid R, Santi DV (2013) Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc Natl Acad Sci 110(6):2318–2323
Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Hp Lee, Lippens E, Duda GN et al (2016) Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15(3):326–334
Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, Kuhl E (2015) Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater 46:318–330
Oyen ML (2008) Poroelastic nanoindentation responses of hydrated bone. J Mater Res 23(5):1307–1314
Mattice JM, Lau AG, Oyen ML, Kent RW (2006) Spherical indentation load-relaxation of soft biological tissues. J Mater Res 21(8):2003–2010
Islam MR, Virag J, Oyen ML (2020) Micromechanical poroelastic and viscoelastic properties of ex-vivo soft tissues. J Biomech 113:110090
Charrier EE, Pogoda K, Wells RG, Janmey PA (2018) Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat Commun 9(1):1–13
Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, Koenderink GH, Weitz DA (2007) The cell as a material. Curr Opin Cell Biol 19(1):101–107
Huang D, Huang Y, Xiao Y, Yang X, Lin H, Feng G, Zhu X, Zhang X (2019) Viscoelasticity in natural tissues and engineered scaffolds for tissue reconstruction. Acta Biomater 97:74–92
Oyen ML (2015) Nanoindentation of hydrated materials and tissues. Curr Opin Solid State Mater Sci 19(6):317–323
Zhao X, Huebsch N, Mooney DJ, Suo Z (2010) Stress-relaxation behavior in gels with ionic and covalent crosslinks. J Appl Phys 107(6):063509
Doehring TC, Carew EO, Vesely I (2004) The effect of strain rate on the viscoelastic response of aortic valve tissue: a direct-fit approach. Ann Biomed Eng 32(2):223–232
Baro VJ, Bonnevie ED, Lai X, Price C, Burris DL, Wang L (2012) Functional characterization of normal and degraded bovine meniscus: rate-dependent indentation and friction studies. Bone 51(2):232–240
Moore A, Zimmerman B, Chen X, Lu X, Burris D (2015) Experimental characterization of biphasic materials using rate-controlled hertzian indentation. Tribol Int 89:2–8
Kuo CK, Li WJ, Mauck RL, Tuan RS (2006) Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol 18(1):64–73
Forte AE, Galvan S, Manieri F, y Baena FR, Dini D, (2016) A composite hydrogel for brain tissue phantoms. Mater Des 112:227–238
Fitzgerald MM, Bootsma K, Berberich JA, Sparks JL (2015) Tunable stress relaxation behavior of an alginate-polyacrylamide hydrogel: comparison with muscle tissue. Biomacromolecules 16(5):1497–1505
Spiller KL, Laurencin SJ, Charlton D, Maher SA, Lowman AM (2008) Superporous hydrogels for cartilage repair: Evaluation of the morphological and mechanical properties. Acta Biomater 4(1):17–25
Oyen ML (2005) Spherical indentation creep following ramp loading. J Mat Res 20(8):2094–2100
Galli M, Comley KS, Shean TA, Oyen ML (2009) Viscoelastic and poroelastic mechanical characterization of hydrated gels. J Mat Res 24(3):973–979
Hu Y, Zhao X, Vlassak JJ, Suo Z (2010) Using indentation to characterize the poroelasticity of gels. Appl Phys Lett 96(12):121904
Strange DG, Fletcher TL, Tonsomboon K, Brawn H, Zhao X, Oyen ML (2013) Separating poroviscoelastic deformation mechanisms in hydrogels. Appl Phys Lett 102(3):031913
Johnson KL (1985) Contact mechanics. Cambridge University Press, Cambridge, UK
Armisen R, Gaiatas F (2009) Agar. Handbook of hydrocolloids, 2nd edn. Elsevier, Boca Raton, pp 82–107
Rowley JA, Madlambayan G, Mooney DJ (1999) Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20(1):45–53
Djabourov M, Leblond J, Papon P (1988) Gelation of aqueous gelatin solutions. i. structural investigation. Journal De Physique 49(2):319–332
Naghash HJ, Okay O (1996) Formation and structure of polyacrylamide gels. J Appl Polym Sci 60(7):971–979
Strange DG, Oyen ML (2012) Composite hydrogels for nucleus pulposus tissue engineering. J Mech Behav Biomed Mater 11:16–26
Kalcioglu ZI, Mahmoodian R, Hu Y, Suo Z, Van Vliet KJ (2012) From macro-to microscale poroelastic characterization of polymeric hydrogels via indentation. Soft Matter 8(12):3393–3398
Lai Y, Hu Y (2018) Probing the swelling-dependent mechanical and transport properties of polyacrylamide hydrogels through afm-based dynamic nanoindentation. Soft Matter 14(14):2619–2627
Tokita M, Tanaka T (1991) Friction coefficient of polymer networks of gels. J Chem Phys 95(6):4613–4619
Islam MR, Oyen ML (2020) A poroelastic master curve for time-dependent and multiscale mechanics of hydrogels. J Mat Res pp 1–9
Oyen ML, Shean TA, Strange DG, Galli M (2012) Size effects in indentation of hydrated biological tissues. J Mat Res 27(1):245–255
Ahearne M, Yang Y, Then KY, Liu KK (2007) An indentation technique to characterize the mechanical and viscoelastic properties of human and porcine corneas. Ann Biomed Eng 35(9):1608–1616
Rubiano A, Qi Y, Guzzo D, Rathinasabapathy A, Rowe K, Pepine C, Simmons C (2016) Stem cell therapy restores viscoelastic properties of myocardium in rat model of hypertension. J Mech Behav Biomed Mater 59:71–77
Qian L, Zhao H, Guo Y, Li Y, Zhou M, Yang L, Wang Z, Sun Y (2018) Influence of strain rate on indentation response of porcine brain. J Mech Behav Biomed Mater 82:210–217
Ahn B, Kim J (2010) Measurement and characterization of soft tissue behavior with surface deformation and force response under large deformations. Med Image Anal 14(2):138–148
Wheatley BB, Fischenich KM, Button KD, Haut RC, Donahue TLH (2015) An optimized transversely isotropic, hyper-poro-viscoelastic finite element model of the meniscus to evaluate mechanical degradation following traumatic loading. J Biomech 48(8):1454–1460
Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Stuart MAC, Boehm H, Li B, Vogel V et al (2012) Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 11(7):642–649
De Gennes PG (1979) Scaling concepts in polymer physics. Cornell University Press, Ithaca, NY
Funding
Funding was provided by the ECU Division of Research, Economic Development and Engagement (REDE) via start-up funds to MLO, including post-doc salary support for MRI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Islam, M.R., Oyen, M.L. Load-Relaxation Characteristics of Chemical and Physical Hydrogels as Soft Tissue Mimics. Exp Mech 61, 939–949 (2021). https://doi.org/10.1007/s11340-021-00712-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-021-00712-x