Abstract
Background
Pulmonary artery hypertension (PAH) is a complex disorder that can lead to right heart failure. The generation of caveolin-1 deficient mice (CAV-1−/−) has provided an alternative genetic model to study the mechanisms of pulmonary hypertension. However, the vascular adaptations in these mice have not been characterized.
Objective
To determine the histological and functional changes in the pulmonary and carotid arteries in CAV-1−/− induced PAH.
Methods
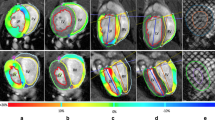
Pulmonary and carotid arteries of young (4–6 months old) and mature (9–12 months old) CAV-1−/− mice were tested and compared to normal wild type mice.
Results
Artery stiffness increases in CAV-1−/− mice, especially the circumferential stiffness of the pulmonary arteries. Increases in stiffness were quantified by a decrease in circumferential stretch and transition strain, increases in elastic moduli, and an increase in total strain energy at physiologic strains. Changes in mechanical properties for the pulmonary artery correlated with increased collagen content while changes in the carotid artery correlated with decreased elastin content.
Conclusions
We demonstrated that an increase in artery stiffness is associated with CAV-1 deficiency-induced pulmonary hypertension. These results improve our understanding of arterial remodeling in PAH.









Similar content being viewed by others
Change history
23 November 2020
A Correction to this paper has been published: https://doi.org/10.1007/s11340-020-00675-5
References
Dodson MW, Brown LM, Elliott CG (2018) Pulmonary arterial hypertension. Heart Fail Clin 14(3):255–269
McLaughlin (2009) ACCF/AHA 2009 Expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association (vol 119, pg 2250, 2009). Circulation 120(2):E13
Wang Z, Chesler NC (2011) Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 1(2):212–223
Thenappan T, Chan SY, Weir EK (2018) Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 315(5):H1322–H1331
Hoffmann J, Marsh LM, Pieper M, Stacher E, Ghanim B, Kovacs G, Konig P, Wilkens H, Haitchi HM, Hoefler G, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G (2015) Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. Am J Physiol Lung Cell Mol Physiol 308(10):L1002–L1013
Wilson JL, Yu J, Taylor L, Polgar P (2015) Hyperplastic growth of pulmonary artery smooth muscle cells from subjects with pulmonary arterial hypertension is activated through JNK and p38 MAPK. PLoS ONE 10(4):e0123662
Gan CTJ, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JWR, Boonstra A, Postmus PE, Vonk-Noordegraaf A (2007) Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132(6):1906–1912
Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J (2012) RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging 5(4):378–387
Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR (2014) Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 4(4):560–580
Morimatsu Y, Sakashita N, Komohara Y, Ohnishi K, Masuda H, Dahan D, Takeya M, Guibert C, Marthan R (2012) Development and characterization of an animal model of severe pulmonary arterial hypertension. J Vasc Res 49(1):33–42
Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF (2009) Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell MolPhysiol 297(6):L1013–L1032
Fung YC, Liu SQ (1991) Changes of zero-stress state of rat pulmonary arteries in hypoxic hypertension. J ApplPhysiol (1985) 70(6):2455–2470
Chen L, Nakano K, Kimura S, Matoba T, Iwata E, Miyagawa M, Tsujimoto H, Nagaoka K, Kishimoto J, Sunagawa K, Egashira K (2011) Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension. Hypertension 57(2):343–350
Wang ZJ, Chesler NC (2012) Role of collagen content and cross-linking in large pulmonary arterial stiffening after chronic hypoxia. Biomech Model Mechanobiol 11(1–2):279–289
Kobs RW, Chesler NC (2006) The mechanobiology of pulmonary vascular remodeling in the congenital absence of eNOS. Biomech Model Mechanobiol 5(4):217–225
Ooi CY, Wang ZJ, Tabima DM, Eickhoff JC, Chesler NC (2010) The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension. Am J Physiol Heart CircPhysiol 299(6):H1823–H1831
Li Z, Huang W, Jiang ZL, Gregersen H, Fung YC (2004) Tissue remodeling of rat pulmonary arteries in recovery from hypoxic hypertension. Proc Natl Acad Sci U S A 101(31):11488–11493
Liu SQ (1997) Regression of hypoxic hypertension-induced changes in the elastic laminae of rat pulmonary arteries. J Appl Physiol (1985) 82(5):1677–1684
Palasiewicz G, Usupbaeva DA, Le Roux H, Sarybayev AS, Plywaczewski R, Mirrakhimov MM, Zielinski J (1998) Three week stay at a height of 3700–4200 m. causes mild pulmonary hypertension in healthy men. PneumonolAlergol Pol 66(11–12):545–550
Zhou Q, Yang S, Luo Y, Qi Y, Yan Z, Shi Z, Fan Y (2012) A randomly-controlled study on the cardiac function at the early stage of return to the plains after short-term exposure to high altitude. PLoS ONE 7(2):e31097
Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J Jr, Chien KR (2002) Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A 99(17):11375–11380
Le Saux O, Teeters K, Miyasato S, Choi J, Nakamatsu G, Richardson JA, Starcher B, Davis EC, Tam EK, Jourdan-Le Saux C (2008) The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am J Physiol Lung Cell MolPhysiol 295(6):L1007–L10017
Achcar RO, Demura Y, Rai PR, Taraseviciene-Stewart L, Kasper M, Voelkel NF, Cool CD (2006) Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest 129(3):696–705
Wang K-Y, Lee M-F, Ho H-C, Liang K-W, Liu C-C, Tsai W-J, Lin W-W (2015) Serum caveolin-1 as a novel biomarker in idiopathic pulmonary artery hypertension. Biomed Res Int 2015:173970
Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP (2006) Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 114(9):912–920
Wunderlich C, Schmeisser A, Heerwagen C, Ebner B, Schober K, Braun-Dullaeus RC, Schwencke C, Kasper M, Morawietz H, Strasser RH (2008) Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther 21(3):507–515
Amin M, Kunkel AG, Le VP, Wagenseil JE (2011) Effect of storage duration on the mechanical behavior of mouse carotid artery. J BiomechEng Trans Asme 133(7):071007
Lee AY, Han B, Lamm SD, Fierro CA, Han HC (2012) Effects of elastin degradation and surrounding matrix support on artery stability. Am J Physiol Heart Circ Physiol 302(4):H873–H884
Moreno J (2012) The effect of pulmonary hypertension on the mechanical properties of arteries in caveolin-1 knockout Mice. In: Mechanical Eng. University of Texas at San Antonio: San Antonio
Ramachandra AB, Humphrey JD (2019) Biomechanical characterization of murine pulmonary arteries. J Biomech 84:18–26
Eberth JF, Cardamone L, Humphrey JD (2011) Evolving biaxial mechanical properties of mouse carotid arteries in hypertension. J Biomech 44(14):2532–2537
Han HC (2009) The mechanical buckling of curved arteries. Mol Cell Biomech 6(2):93–99
Lee AY, Han HC (2010) A Nonlinear Thin-Wall Model for Vein Buckling. Cardiovasc Eng 1(4):282–289
Chesler NC, Thompson-Figueroa J, Millburne K (2004) Measurements of mouse pulmonary artery biomechanics. J Biomech Eng 126(2):309–314
Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R (2008) Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. American Journal of Physiology-Heart and Circulatory Physiology 295(4):H1451–H1459
Bellofiore A, Henningsen J, Lepak CG, Tian L, Roldan-Alzate A, Kellihan HB, Consigny DW, Francois CJ, Chesler NC (2015) A novel in vivo approach to assess radial and axial distensibility of large and intermediate pulmonary artery branches. J Biomech Eng 137(4):044501
Fung YC (1981) Biomechanics: mechanical properties of living tissues Springer-Verlag, New York. xii, 433 p
Hu JJ, Baek S, Humphrey JD (2007) Stress-strain behavior of the passive basilar artery in normotension and hypertension. J Biomech 40(11):2559–2563
Garcia JR, Lamm SD, Han HC (2013) Twist buckling behavior of arteries. Biomech Model Mechanobiol 12(5):915–927
Qi N, Gao H, Ogden RW, Hill NA, Holzapfel GA, Han HC, Luo X (2015) Investigation of the optimal collagen fibre orientation in human iliac arteries. J Mech Behav Biomed Mater 52:108–119
Wagenseil JE, Mecham RP (2009) Vascular extracellular matrix and arterial mechanics. Physiol Rev 89(3):957–989
Albinsson S, Shakirova Y, Rippe A, Baumgarten M, Rosengren BI, Rippe C, Hallmann R, Hellstrand P, Rippe B, Sward K (2007) Arterial remodeling and plasma volume expansion in caveolin-1-deficient mice. Am J Physiol Regul Integr Comp Physiol 293(3):R1222–R1231
Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC (2007) Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204(10):2373–2382
Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293(5539):2449–2452
Tabima DM, Chesler NC (2010) The effects of vasoactivity and hypoxic pulmonary hypertension on extralobar pulmonary artery biomechanics. J Biomech 43(10):1864–1869
Kobs RW, Muvarak NE, Eickhoff JC, Chesler NC (2005) Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. American Journal of Physiology-Heart and Circulatory Physiology 288(3):H1209–H1217
Golob MJ, Tabima DM, Wolf GD, Johnston JL, Forouzan O, Mulchrone AM, Kellihan HB, Bates ML, Chesler NC (2017) Pulmonary arterial strain- and remodeling-induced stiffening are differentiated in a chronic model of pulmonary hypertension. J Biomech 55:92–98
Todorovich-Hunter L, Johnson DJ, Ranger P, Keeley FW, Rabinovitch M (1988) Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest 58(2):184–195
Fonck E, Prod’hom G, Roy S, Augsburger L, Rufenacht DA, Stergiopulos N (2007) Effect of elastin degradation on carotid wall mechanics as assessed by a constituent-based biomechanical model. American Journal of Physiology-Heart and Circulatory Physiology 292(6):H2754–H2763
Wagenseil JE, Mecham RP (2012) Elastin in large artery stiffness and hypertension. J CardiovascTransl Res 5(3):264–273
Martinez R, Han HC (2012) The effect of collagenase on the critical buckling pressure of arteries. Mol Cell Biomech 9(1):55–75
Collins MJ, Eberth JF, Wilson E, Humphrey JD (2012) Acute mechanical effects of elastase on the infrarenal mouse aorta: implications for models of aneurysms. J Biomech 45(4):660–665
Wang GL, Wang LY, Yang SX, Zhang P, Chen XH, Yao QP, Gong XB, Qi YX, Jiang ZL, Han HC (2017) Arterial wall remodeling under sustained axial twisting in rats. J Biomech 60:124–133
Rahman A, Sward K (2009) The role of caveolin-1 in cardiovascular regulation. Acta Physiol (Oxf) 195(2):231–245
Sward K, Albinsson S, Rippe C (2014) Arterial dysfunction but maintained systemic blood pressure in cavin-1-deficient mice. PLoS ONE 9(3):e92428
Han HC (2008) Nonlinear buckling of blood vessels: a theoretical study. J Biomech 41(12):2708–2713
Huang W, Delgado-West D, Wu JT, Fung YC (2001) Tissue remodeling of rat pulmonary artery in hypoxic breathing. II. Course of change of mechanical properties. Ann Biomed Eng 29(7):552–562
Acknowledgments
This work was partially supported by the National Institutes of Health HL095852 (HCH), NHLBI HHSN 268201000036C (N01-HV-00244) for the UTHSCSA Cardiovascular Proteomics Center, and the Janey Briscoe Center of Excellence in Cardiovascular Research (CJLS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
All authors have not conflict of interest.
Human and Animal Rights
This study did not involve any human subject and the use of lab animal followed a protocol which minimized the use of animal and fully considered the welfare of the animal was approved by IACUC.
Additional information
The original article was updated to include the missing section from equation (4) to equation (8). This error was introduced during production process and was not the fault of author.
Rights and permissions
About this article
Cite this article
Moreno, J., Escobedo, D., Calhoun, C. et al. Arterial Wall Stiffening in Caveolin-1 Deficiency-Induced Pulmonary Artery Hypertension in Mice. Exp Mech 61, 217–228 (2021). https://doi.org/10.1007/s11340-020-00666-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-020-00666-6