Abstract
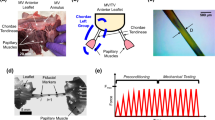
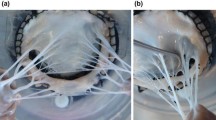
Background: Tricuspid valve chordae tendineae play a vital role in our cardiovascular system. They function as “parachute cords” to the tricuspid leaflets to prevent prolapse during systole. However, in contrast to the tricuspid annulus and leaflets, the tricuspid chordae tendineae have received little attention. Few previous studies have described their mechanics and their structure-function relationship. Objective: In this study, we aimed to quantify the mechanics of tricuspid chordae tendineae based on their leaflet of origin, insertion site, and size. Methods: Specifically, we uniaxially stretched 53 tricuspid chordae tendineae from sheep and recorded their stress-strain behavior. We also analyzed the microstructure of the tricuspid chordae tendineae based on two-photon microscopy and histology. Finally, we compared eight different hyperelastic constitutive models and their ability to fit our data. Results: We found that tricuspid chordae tendineae are highly organized collageneous tissues, which are populated with cells throughout their thickness. In uniaxial stretching, this microstructure causes the classic J-shaped nonlinear stress-strain response known from other collageneous tissues. We found differences in stiffness between tricuspid chordae tendineae from the anterior, posterior, or septal leaflets only at small strains. Similarly, we found significant differences based on their insertion site or size also only at small strains. Of the models we fit to our data, we recommend the Ogden two-parameter model. This model fit the data excellently and required a minimal number of parameters. For future use, we identified and reported the Ogden material parameters for an average data set. Conclusion: The data presented in this study help to explain the mechanics and structure-function relationship of tricuspid chordae tendineae and provide a model recommendation (with parameters) for use in computational simulations of the tricuspid valve.





Similar content being viewed by others
References
Sacks MS, Yoganathan AP (2007) . Philos Trans R Soc B: Biol Sciences 362 (1484):1369. https://doi.org/10.1098/rstb.2007.2122
Meador WD, Mathur M, Rausch M (2018) Advances in Heart Valve Biomechanics, pp 105–114. https://doi.org/10.1007/978-3-030-01993-8_5
Cevasco M, Shekar PS (2017) . Ann Cardiothoracic Surg 6(3):275
Mascherbauer J, Maurer G (2010) . Eur Heart J 31(23):2841. https://doi.org/10.1093/eurheartj/ehq303
Oliveira DC, Oliveira CG (2019) . Cardiol Res 10(4):199. https://doi.org/10.14740/cr874
Lee CH, Laurence D, Ross C, Kramer KE, Babu AR, Johnson EL, Hsu MC, Aggarwal A, Mir A, Burkhart HM, Towner R, Baumwart R, Wu Y, Lee CH, Laurence D, Ross C, Kramer KE, Babu AR, Johnson EL, Hsu MC, Aggarwal A, Mir A, Burkhart HM, Towner R, Baumwart R, Wu Y (2019) . Bioengineering 6(2):47. https://doi.org/10.3390/bioengineering6020047
Stevanella M, Votta E, Lemma M, Antona C, Redaelli A (2010) . Med Eng Phys 32(10):1213. https://doi.org/10.1016/j.medengphy.2010.08.013
Kong F, Pham T, Martin C, McKay R, Primiano C, Hashim S, Kodali S, Sun W (2018) . Ann Biomed Eng 46(8):1112. https://doi.org/10.1007/s10439-018-2024-8
Dabiri Y, Yao J, Sack KL, Kassab GS, Guccione JM (2019) . Mech Res Commun 97:96. https://doi.org/10.1016/j.mechrescom.2019.04.009
Singh-Gryzbon S, Sadri V, Toma M, Pierce EL, Wei ZA, Yoganathan AP (2019) Annals of Biomedical Engineering. https://doi.org/10.1007/s10439-019-02243-y
Lim KO (1980) . Jpn J Physiol 30(3):455. https://doi.org/10.2170/jjphysiol.30.455
Lim KO, Boughner DR, Perkins DG (1983) . Jpn Heart J 24(4):539. https://doi.org/10.1536/ihj.24.539
Pokutta-Paskaleva A, Sulejmani F, DelRocini M, Sun W (2019) . Acta Biomaterialia 85:241. https://doi.org/10.1016/j.actbio.2018.12.029
Dixon JA, Spinale FG (2009) . Circ Heart Fail 2(3):262. https://doi.org/10.1161/CIRCHEARTFAILURE.108.814459
Meador WD, Mathur M, Sugerman GP, Jazwiec T, Malinowski M, Bersi MR, Timek TA, Rausch M (2019) Acta Biomaterialia. https://doi.org/10.1016/j.actbio.2019.11.039
Meador WD, Sugerman GP, Story HM, Seifert AW, Bersi MR, Tepole AB, Rausch M (2019) Acta Biomaterialia (xxxx). https://doi.org/10.1016/j.actbio.2019.10.020
Rezakhaniha R, Agianniotis A, Schrauwen JTC, Griffa A, Sage D, Bouten CVC, van de Vosse FN, Unser M, Stergiopulos N (2012) . Biomech Model Mechanobiol 11(3-4):461
Ogden RW, Saccomandi G, Sgura I (2004) . Comput Mech 34(6):484. https://doi.org/10.1007/s00466-004-0593-y
Singmann H, Bolker B, Westfall J (2015) Analysis of Factorial Experiments, package ‘afex’
Enriquez-Sarano M, Messika-Zeitoun D, Topilsky Y, Tribouilloy C, Benfari G, Michelena H (2019) Progress in cardiovascular diseases
Mathur M, Jazwiec T, Meador WD, Malinowski M, Goehler M, Ferguson H, Timek TA, Rausch M (2019) Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-019-01148-y
Khoiy K, Biswas D, Decker TN, Asgarian KT, Loth F, Amini R (2016) J Biomech Eng 138(11):111006. https://doi.org/10.1115/1.4034621
Pham T, Sulejmani F, Shin E, Wang D, Sun W (2017) . Acta Biomater 54:345. https://doi.org/10.1016/j.actbio.2017.03.026
Laurence D, Ross C, Jett S, Johns C, Echols A, Baumwart R, Towner R, Liao J, Bajona P, Wu Y, Lee CH (2019) . J Biomech 83:16. https://doi.org/10.1016/j.jbiomech.2018.11.015
Spinner EM, Shannon P, Buice D, Jimenez JH, Veledar E, Del Nido PJ, Adams DH, Yoganathan AP (2011) . Circulation 124(8):920. https://doi.org/10.1161/CIRCULATIONAHA.110.003897
Rausch M, Malinowski M, Meador WD, Wilton P, Khaghani A, Timek TA (2018) . Cardiovascul Eng Technol 9(3):365. https://doi.org/10.1007/s13239-018-0367-9
Malinowski M, Jazwiec T, Goehler M, Quay M, Bush J, Jovinge S, Rausch M, Timek T (2018) Journal of Thoracic and Cardiovascular Surgery. https://doi.org/10.1016/j.jtcvs.2018.08.110
Rausch M, Mathur M, Meador WD (2019) GAMM-Mitteilungen, pp e201900012. https://doi.org/10.1002/gamm.201900012
Rausch M (2020) Current opinion in biomedical engineering
Troxler LG, Spinner EM, Yoganathan AP (2012) . J Biomech 45(6):1084
Humphrey JD (2003) . Proc R Soc Math Phys Eng Sci 459(2029):3. https://doi.org/10.1098/rspa.2002.1060
Mow VC, Hou JS, Owens JM, Ratcliffe A (1990) Biomechanics of Diarthrodial Joints. Springer, New York, pp 215–260. https://doi.org/10.1007/978-1-4612-3448-7_8
Fung YC (1967) . Amer J Physiol 213(6):1532. https://doi.org/10.1152/ajplegacy.1967.213.6.1532
Holzapfel GA, Gasser TC, Ogden RW (2000) . J Elast 61(1/3):1
Gasser TC, Ogden RW, Holzapfel GA (2006) . J R Soc Interface 3 (6):15. https://doi.org/10.1098/rsif.2005.0073
Maas SA, Ellis BJ, Ateshian GA, Weiss JA (2012) J Biomech Eng 134(1):011005. https://doi.org/10.1115/1.4005694
Dassault Systemes (SIMULIA) (2014) Providence, RI. Abaqus 6.14 Documentation
Duginski GA, Ross C, Laurence D, Johns CH, Lee CH (2020) J Mech Behav Biomed Mater 101(July 2019). https://doi.org/10.1016/j.jmbbm.2019.103438
Salinas SD, Clark MM, Amini R (2019) Journal of Biomechanics (xxxx), pp 109462. https://doi.org/10.1016/j.jbiomech.2019.109462
Acknowledgments
This work was supported by the American Heart Association under Award 18CDA34120028 and the National Institutes of Health under Award 1 F31 HL145976-01A1. Dr. Rausch has a speaking agreement with Edwards Lifesciences. No other author has any conflicts to report. We performed all experimental and animal procedures in congruence with the Guide for Care and Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institutes of Health, and the Principles of Laboratory Animal Care, formulated by the National Society for Medical Research. The study protocol was approved by our local Institutional Animal Care and Use Committee (Spectrum Health IACUC No.: 18-01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Smith, K.J., Mathur, M., Meador, W.D. et al. Tricuspid Chordae Tendineae Mechanics: Insertion Site, Leaflet, and Size-Specific Analysis and Constitutive Modelling. Exp Mech 61, 19–29 (2021). https://doi.org/10.1007/s11340-020-00594-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-020-00594-5