Abstract
Purpose
Previous observational studies have suggested an association between sleep disturbance and metabolic syndrome (MetS). However, it remains unclear whether this association is causal. This study aims to investigate the causal effects of sleep-related traits on MetS using Mendelian randomization (MR).
Methods
Single-nucleotide polymorphisms strongly associated with daytime napping, insomnia, chronotype, short sleep, and long sleep were selected as genetic instruments from the corresponding genome-wide association studies (GWAS). Summary-level data for MetS were obtained from two independent GWAS datasets. Univariable and multivariable MR analyses were conducted to investigate and verify the causal effects of sleep traits on MetS.
Results
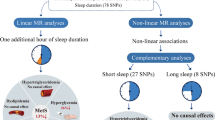
The univariable MR analysis demonstrated that genetically predicted daytime napping and insomnia were associated with increased risk of MetS in both discovery dataset (OR daytime napping = 1.630, 95% CI 1.273, 2.086; OR insomnia = 1.155, 95% CI 1.108, 1.204) and replication dataset (OR daytime napping = 1.325, 95% CI 1.131, 1.551; OR insomnia = 1.072, 95% CI 1.046, 1.099). For components, daytime napping was positively associated with triglycerides (beta = 0.383, 95% CI 0.160, 0.607) and waist circumference (beta = 0.383, 95% CI 0.184, 0.583). Insomnia was positively associated with hypertension (OR = 1.101, 95% CI 1.042, 1.162) and waist circumference (beta = 0.067, 95% CI 0.031, 0.104). The multivariable MR analysis indicated that the adverse effect of daytime napping and insomnia on MetS persisted after adjusting for BMI, smoking, drinking, and another sleep trait.
Conclusion
Our study supported daytime napping and insomnia were potential causal factors for MetS characterized by central obesity, hypertension, or elevated triglycerides.


Similar content being viewed by others
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
Eckel RH, Alberti K, Grundy SM, Zimmet PZ (2010) The metabolic syndrome. Lancet 375:181–183. https://doi.org/10.1016/S0140-6736(09)61794-3
Pammer LM, Lamina C, Schultheiss UT et al (2021) Association of the metabolic syndrome with mortality and major adverse cardiac events: a large chronic kidney disease cohort. J Intern Med 290:1219–1232. https://doi.org/10.1111/joim.13355
Saklayen MG (2018) The global epidemic of the metabolic syndrome. Curr Hypertens Rep 20:12. https://doi.org/10.1007/s11906-018-0812-z
Hirode G, Wong RJ (2020) Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA 323:2526. https://doi.org/10.1001/jama.2020.4501
Jia Y, Guo D, Sun L et al (2022) Self-reported daytime napping, daytime sleepiness, and other sleep phenotypes in the development of cardiometabolic diseases: a Mendelian randomization study. Eur J Prev Cardiol 29:1982–1991. https://doi.org/10.1093/eurjpc/zwac123
Sun J, Ma C, Zhao M et al (2022) Daytime napping and cardiovascular risk factors, cardiovascular disease, and mortality: a systematic review. Sleep Med Rev 65:101682. https://doi.org/10.1016/j.smrv.2022.101682
Che T, Yan C, Tian D et al (2021) The association between sleep and metabolic syndrome: a systematic review and meta-analysis. Front Endocrinol 12:773646. https://doi.org/10.3389/fendo.2021.773646
Deng H-B, Tam T, Zee BC-Y et al (2017) Short sleep duration increases metabolic impact in healthy adults: a population-based cohort study. Sleep 40:zsx130. https://doi.org/10.1093/sleep/zsx130
Garbarino S, Magnavita N (2019) Sleep problems are a strong predictor of stress-related metabolic changes in police officers. A prospective study. PLoS ONE 14:e0224259. https://doi.org/10.1371/journal.pone.0224259
Yu JH, Yun C-H, Ahn JH et al (2015) Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab 100:1494–1502. https://doi.org/10.1210/jc.2014-3754
Vizmanos B, Cascales AI, Rodríguez-Martín M et al (2023) Lifestyle mediators of associations among siestas, obesity, and metabolic health. Obes Silver Spring Md 31:1227–1239. https://doi.org/10.1002/oby.23765
Choi S, Kim K, Lee JK et al (2019) Association between change in alcohol consumption and metabolic syndrome: analysis from the health examinees study. Diabetes Metab J 43:615–626. https://doi.org/10.4093/dmj.2018.0128
Yao F, Bo Y, Zhao L et al (2021) Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients 13:4475. https://doi.org/10.3390/nu13124475
Wen L, Fan J, Shi X, et al (2024) Causal association of rheumatoid arthritis with frailty and the mediation role of inflammatory cytokines: a Mendelian randomization study. Arch Gerontol Geriatr 105348. https://doi.org/10.1016/j.archger.2024.105348
Dashti HS, Daghlas I, Lane JM et al (2021) Genetic determinants of daytime napping and effects on cardiometabolic health. Nat Commun 12:900. https://doi.org/10.1038/s41467-020-20585-3
Dashti HS, Jones SE, Wood AR et al (2019) Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun 10:1100. https://doi.org/10.1038/s41467-019-08917-4
Jansen PR, Watanabe K, Stringer S et al (2019) Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet 51:394–403. https://doi.org/10.1038/s41588-018-0333-3
Jones SE, Lane JM, Wood AR et al (2019) Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun 10:343. https://doi.org/10.1038/s41467-018-08259-7
Lind L (2019) Genome-wide association study of the metabolic syndrome in UK Biobank. Metab Syndr Relat Disord 17:505–511. https://doi.org/10.1089/met.2019.0070
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285:2486–2497. https://doi.org/10.1001/jama.285.19.2486
Burgess S, Davies NM, Thompson SG (2016) Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 40:597–608. https://doi.org/10.1002/gepi.21998
van Walree ES, Jansen IE, Bell NY et al (2022) Disentangling genetic risks for metabolic syndrome. Diabetes 71:2447–2457. https://doi.org/10.2337/db22-0478
Shungin D, Winkler TW, Croteau-Chonka DC et al (2015) New genetic loci link adipose and insulin biology to body fat distribution. Nature 518:187–196. https://doi.org/10.1038/nature14132
Kurki MI, Karjalainen J, Palta P et al (2023) FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613:508–518. https://doi.org/10.1038/s41586-022-05473-8
Willer CJ, Schmidt EM, Sengupta S et al (2013) Discovery and refinement of loci associated with lipid levels. Nat Genet 45:1274–1283. https://doi.org/10.1038/ng.2797
Chen J, Spracklen CN, Marenne G et al (2021) The trans-ancestral genomic architecture of glycemic traits. Nat Genet 53:840–860. https://doi.org/10.1038/s41588-021-00852-9
Liu M, Jiang Y, Wedow R et al (2019) Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51:237–244. https://doi.org/10.1038/s41588-018-0307-5
Yengo L, Sidorenko J, Kemper KE et al (2018) Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet 27:3641–3649. https://doi.org/10.1093/hmg/ddy271
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665. https://doi.org/10.1002/gepi.21758
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525. https://doi.org/10.1093/ije/dyv080
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314. https://doi.org/10.1002/gepi.21965
Verbanck M, Chen C-Y, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50:693–698. https://doi.org/10.1038/s41588-018-0099-7
Tse LA, Wang C, Rangarajan S et al (2021) Timing and length of nocturnal sleep and daytime napping and associations with obesity types in high-, middle-, and low-income countries. JAMA Netw Open 4:e2113775. https://doi.org/10.1001/jamanetworkopen.2021.13775
Cai H, Su N, Li W et al (2021) Relationship between afternoon napping and cognitive function in the ageing Chinese population. Gen Psychiatry 34:e100361. https://doi.org/10.1136/gpsych-2020-100361
Zou D, Wennman H, Hedner J et al (2021) Insomnia is associated with metabolic syndrome in a middle-aged population: the SCAPIS pilot cohort. Eur J Prev Cardiol 28:e26–e28. https://doi.org/10.1177/2047487320940862
Syauqy A, Hsu C-Y, Rau H-H et al (2019) Association of sleep duration and insomnia symptoms with components of metabolic syndrome and inflammation in middle-aged and older adults with metabolic syndrome in Taiwan. Nutrients 11:1848. https://doi.org/10.3390/nu11081848
Vera B, Dashti HS, Gómez-Abellán P, et al (2018) Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci Rep 8. https://doi.org/10.1038/s41598-017-18268-z
Devine JK, Wolf JM (2016) Determinants of cortisol awakening responses to naps and nighttime sleep. Psychoneuroendocrinology 63:128–134. https://doi.org/10.1016/j.psyneuen.2015.09.016
Anagnostis P, Athyros VG, Tziomalos K et al (2009) The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701. https://doi.org/10.1210/jc.2009-0370
Funding
This research was supported by the National Natural Science Foundation of China (No.82304251).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
We used publicly available GWAS summary statistics and each GWAS was approved by its corresponding ethics committee and followed the tenants of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Wen, L., Shi, X. et al. Causal effects of sleep traits on metabolic syndrome and its components: a Mendelian randomization study. Sleep Breath (2024). https://doi.org/10.1007/s11325-024-03020-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11325-024-03020-5