Abstract
Purpose
To aim of the study was to explore the possible mechanisms for the decreased contraction capacity of the palatopharyngeal muscle in cases with obstructive sleep apnea hypopnea syndrome (OSAHS).
Methods
Palatopharyngeal muscle specimens from patients with OSAHS were taken as the case group. Palatopharyngeal muscle tissue by surgical removal of oropharyngeal malignant tumors was used as a control cohort. The palatopharyngeal muscle contraction capacity was measured by assessing diaphragm peak-twitching force / cross-sectional area (Pt/CSA), fatigue index (FI) twitch tension, and force per cross-sectional area (Force/CSA). Myofibril and sarcoplasmic reticulum (SR) ultra-structures were observed by electron microscopy. The intra-cellular calcium concentration was measured by fluorescence spectrophotometry. DHPRα1s and RyR1 expression profiles were probed through RT-qPCR and Western blot, and the colocalization of them was determined by immunofluorescence.
Results
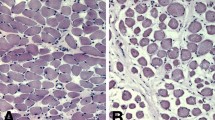
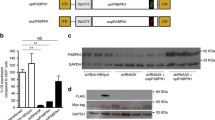
In comparison with the control cohort, the OSAHS cohort demonstrated decreased Pt/CSA (P < 0.01), FI twitch tension (P < 0.01), together with contraction capacity (P < 0.01). This cohort also had lower intra-cellular [Ca2+] of palatopharyngeal muscle cells with abnormal ultrastructure of sarcoplasmic reticulum (SR) (P < 0.01). In addition, transcriptomic (P < 0.01) and proteomic expression (P < 0.01) for RyR1 and DHPRα1s were markedly reduced within OSAHS cohort, although the degree of colocalization of them was not altered.
Conclusion
RyR1 and DHPRα1s downregulation may disrupt intra-cellular [Ca2+] homeostasis and subsequently decrease the palatopharyngeal muscle contraction capacity in patients with OSAHS, thus providing a novel insight into the pathogenesis of OSAHS.





Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- BSA:
-
Bovine serum albumin
- CSA:
-
Cross-sectional area
- DHPRa1s:
-
Dihydropyridine receptors
- DMEM:
-
Dulbecco’s modified Eagle medium
- ECC:
-
Excitation–contraction coupling
- EM:
-
Electron microscopy
- FI:
-
Fatigue index
- IF:
-
Immunofluorescence
- L0 :
-
Length of the muscle produced maximal isometric tension
- NMF:
-
Normal myofibril
- NSR:
-
Normal sarcoplasmic reticulum
- OSAHS:
-
Obstructive sleep apnea hypopnea syndrome
- P0 :
-
Maximal tetanic force
- PBS:
-
Phosphate-buffered saline
- Pt:
-
Peak twitch force
- RyR1:
-
Ryanodine receptor
- SER:
-
Smooth endoplasmic reticulum
- SR:
-
Sarcoplasmic reticulum
- Vv :
-
Volume fraction
References
Lee JJ, Sundar KM (2021) Evaluation and Management of Adults with Obstructive Sleep Apnea Syndrome. Lung 199(2):87–101
Mohit A, Shrivastava P (2021) Chand, Molecular determinants of obstructive sleep apnea. Sleep Med 80:105–112
Ko CY, Liu QQ, Su HZ, Zhang HP, Fan JM, Yang JH, Hu AK, Liu YQ, Chou D, Zeng YM (2019) Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin Sci (Lond) 133(7):905–917
Garg RK, Afifi AM, Sanchez R, King TW (2016) Obstructive Sleep Apnea in Adults: The Role of Upper Airway and Facial Skeletal Surgery. Plast Reconstr Surg 138(4):889–898
Zhan X, Li L, Wu C, Chitguppi C, Huntley C, Fang F, Wei Y (2019) Effect of uvulopalatopharyngoplasty (UPPP) on atherosclerosis and cardiac functioning in obstructive sleep apnea patients. Acta Otolaryngol 139(9):793–797
Eshima H, Poole DC, Kano Y (2014) In vivo calcium regulation in diabetic skeletal muscle. Cell Calcium 56(5):381–389
Allen DG, Lamb GD, Westerblad H (2008) Impaired calcium release during fatigue. J Appl Physiol (1985) 104(1):296–305
Luo Y, Rall JA (2006) Regulation of contraction kinetics in skinned skeletal muscle fibers by calcium and troponin C. Arch Biochem Biophys 456(2):119–126
Kushnir A, Wajsberg B, Marks AR (2018) Ryanodine receptor dysfunction in human disorders. Biochim Biophys Acta Mol Cell Res 1865(11 Pt B):1687–1697
Clancy JS, Takeshima H, Hamilton SL, Reid MB (1999) Contractile function is unaltered in diaphragm from mice lacking calcium release channel isoform 3. Am J Physiol 277(4 Pt 2):R1205–R1209
Fill M, Copello JA (2002) Ryanodine receptor calcium release channels. Physiol Rev 82(4):893–922
Ogawa Y, Kurebayashi N, Murayama T (2000) Putative roles of type 3 ryanodine receptor isoforms (RyR3). Trends Cardiovasc Med 10(2):65–70
Dulhunty AF, Wei-LaPierre L, Casarotto MG, Beard NA (2017) Core skeletal muscle ryanodine receptor calcium release complex. Clin Exp Pharmacol Physiol 44(1):3–12
Wang MM, Hao LY, Guo F, Zhong B, Zhong XM, Yuan J, Hao YF, Zhao S, Sun XF, Lei M, Jiao GY (2017) Decreased intracellular [Ca(2+) ] coincides with reduced expression of Dhpralpha1s, RyR1, and diaphragmatic dysfunction in a rat model of sepsis. Muscle Nerve 56(6):1128–1136
Jiao GY, Hao LY, Gao CE, Chen L, Sun XF, Yang HL, Li Y, Dai YN (2013) Reduced DHPRalpha1S and RyR1 expression levels are associated with diaphragm contractile dysfunction during sepsis. Muscle Nerve 48(5):745–751
Du K, Liu M, Zhong X, Yao W, Xiao Q, Wen Q, Yang B, Wei M (2018) Epigallocatechin Gallate Reduces Amyloid beta-Induced Neurotoxicity via Inhibiting Endoplasmic Reticulum Stress-Mediated Apoptosis. Mol Nutr Food Res 62(8):e1700890
Tangel DJ, Mezzanotte WS, White DP (1991) Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J Appl Physiol (1985) 70(6):2574–81
Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP (2000) Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med 161(5):1746–1749
Dong J, Niu X, Chen X (2020) Injury and Apoptosis in the Palatopharyngeal Muscle in Patients with Obstructive Sleep Apnea-Hypopnea Syndrome. Med Sci Monit 26:e919501
Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ (2004) Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 170(5):541–546
Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E (1998) Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med 157(2):586–593
Series F, Simoneau JA, St Pierre S (2000) Muscle fiber area distribution of musculus uvulae in obstructive sleep apnea and non-apneic snorers. Int J Obes Relat Metab Disord 24(4):410–5
Lynch PJ, Tong J, Lehane M, Mallet A, Giblin L, Heffron JJ, Vaughan P, Zafra G, MacLennan DH, McCarthy TV (1999) A mutation in the transmembrane/luminal domain of the ryanodine receptor is associated with abnormal Ca2+ release channel function and severe central core disease. Proc Natl Acad Sci U S A 96(7):4164–4169
Avila G, Dirksen RT (2001) Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol 118(3):277–290
Andersson DC, Meli AC, Reiken S, Betzenhauser MJ, Umanskaya A, Shiomi T, D’Armiento J, Marks AR (2012) Leaky ryanodine receptors in beta-sarcoglycan deficient mice: a potential common defect in muscular dystrophy. Skelet Muscle 2(1):9
Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15(3):325–330
Samso M (2015) 3D Structure of the Dihydropyridine Receptor of Skeletal Muscle. Eur J Transl Myol 25(1):4840
Cheng W, Altafaj X, Ronjat M, Coronado R (2005) Interaction between the dihydropyridine receptor Ca2+ channel beta-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proc Natl Acad Sci U S A 102(52):19225–19230
FleuryCurado T, Pho H, Freire C, Amorim MR, Bonaventura J, Kim LJ, Lee R, Cabassa ME, Streeter SR, Branco LG, Sennes LU, Fishbein K, Spencer RG, Schwartz AR, Brennick MJ, Michaelides M, Fuller DD, Polotsky VY (2021) Designer Receptors Exclusively Activated by Designer Drugs Approach to Treatment of Sleep-disordered Breathing. Am J Respir Crit Care Med 203(1):102–110
Perger E, Taranto-Montemurro L (2021) Upper airway muscles: influence on obstructive sleep apnoea pathophysiology and pharmacological and technical treatment options. Curr Opin Pulm Med 27(6):505–513
Funding
This study was supported by Youth backbone support Program of China Medical University (QGJ2018061).
Author information
Authors and Affiliations
Contributions
KD, LH and MW conceived and designed the experiments. MW, QZ, ZT M and HY performed the examination and prepared Figures. KD and LH wrote the manuscript; KD, LH and MW reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
None of the authors has any conflict of interest to disclose.
Ethics approval and consent to participate
Ethical approval was obtained from the ethics committee of Shengjing Hospital of China Medical University (2020PS363K(X1)) and informed consent obtained from all participants.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, M., Zhao, Q., Ma, Z. et al. The reduced contraction capacity of palatopharyngeal muscle in OSAHS is related to the decreased intra-cellular [Ca2+] mediated by low RyR1 and DHPRα1s expression. Sleep Breath 26, 1791–1799 (2022). https://doi.org/10.1007/s11325-022-02562-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-022-02562-w