Abstract
Purpose
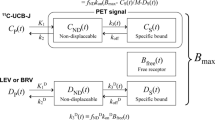
PET imaging using [11C]metoclopramide revealed the importance of P-glycoprotein (P-gp, ABCB1) in mediating the brain-to-blood efflux of substrates across the blood–brain barrier (BBB). In this work, the elimination rate constant from the brain (kE,brain), calculated from dynamic PET images without the need for arterial blood sampling, was evaluated as an outcome parameter for the interpretation of [11C]metoclopramide PET data.
Procedures
kE,brain parameter was obtained by linear regression of log-transformed brain time-activity curves (TACs). kE,brain values (h−1) obtained under baseline conditions were compared with values obtained after complete P-gp inhibition using tariquidar in rats (n = 4) and baboons (n = 4) or after partial inhibition using cyclosporine A in humans (n = 10). In baboons, the sensitivity of kE,brain to measure complete P-gp inhibition was compared with outcome parameters derived from kinetic modeling using a 1-tissue compartment model (1-TCM). Finally, kE,brain-maps were generated in each species using PMOD software.
Results
The linear part of the log-transformed brain TACs occurred from 10 to 30 min after radiotracer injection in rats, from 15 to 60 min in baboons, and from 20 to 60 min in humans. P-gp inhibition significantly decreased kE,brain values by 39 ± 12% in rats (p < 0.01), by 32 ± 6% in baboons (p < 0.001), and by 37 ± 22% in humans (p < 0.001). In baboons, P-gp inhibition consistently decreased the brain-to-plasma efflux rate constant k2 (36 ± 9%, p < 0.01) leading to an increase in the total brain volume of distribution (VT, 101 ± 12%, p < 0.001). In all studied species, brain kE,brain-maps displayed decreased P-gp-mediated efflux across the BBB.
Conclusions
kE,brain of [11C]metoclopramide provides a simple outcome parameter to describe P-gp function in the living brain when arterial input function data are unavailable, although less sensitive than VT. kE,brain-maps represent easy to compute parametric images reflecting the effect of P-gp on [11C]metoclopramide elimination from the brain.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abbott NJ, Patabendige AAK, Dolman DEM et al (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Kannan P, John C, Zoghbi SS et al (2009) Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther 86:368–377. https://doi.org/10.1038/clpt.2009.138
Langer O (2016) Use of PET imaging to evaluate transporter-mediated drug-drug interactions. J Clin Pharmacol 56(Suppl 7):S143-156. https://doi.org/10.1002/jcph.722
Deo AK, Borson S, Link JM et al (2014) Activity of P-glycoprotein, a β-amyloid transporter at the blood-brain barrier, is compromised in patients with mild Alzheimer’s disease. J Nucl Med 55:1106–1111. https://doi.org/10.2967/jnumed.113.130161
Kortekaas R, Leenders KL, van Oostrom JCH et al (2005) Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57:176–179. https://doi.org/10.1002/ana.20369
Lubberink M (2016) Kinetic models for measuring P-glycoprotein function at the blood-brain barrier with positron emission tomography. Curr Pharm Des 22:5786–5792. https://doi.org/10.2174/1381612822666160804093852
Tournier N, Stieger B, Langer O (2018) Imaging techniques to study drug transporter function in vivo. Pharmacol Ther 189:104–122. https://doi.org/10.1016/j.pharmthera.2018.04.006
Muzi M, Mankoff DA, Link JM et al (2009) Imaging of cyclosporine inhibition of P-glycoprotein activity using 11C-verapamil in the brain: studies of healthy humans. J Nucl Med 50:1267–1275. https://doi.org/10.2967/jnumed.108.059162
Bauer M, Zeitlinger M, Karch R et al (2012) P-glycoprotein mediated interaction between (R)-[11C]verapamil and tariquidar at the human blood-brain barrier studied with positron emission tomography, a comparison with rat data. Clin Pharmacol Ther 91:227–233. https://doi.org/10.1038/clpt.2011.217
Kreisl WC, Liow J-S, Kimura N et al (2010) P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med 51:559–566. https://doi.org/10.2967/jnumed.109.070151
Tournier N, Bauer M, Pichler V et al (2019) Impact of P-glycoprotein function on the brain kinetics of the weak substrate 11C-metoclopramide assessed with PET imaging in humans. J Nucl Med 60:985–991. https://doi.org/10.2967/jnumed.118.219972
Zoufal V, Mairinger S, Brackhan M et al (2020) Imaging P-glycoprotein induction at the blood-brain barrier of a β-amyloidosis mouse model with 11C-metoclopramide PET. J Nucl Med 61:1050–1057. https://doi.org/10.2967/jnumed.119.237198
Pottier G, Marie S, Goutal S et al (2016) Imaging the impact of the P-glycoprotein (ABCB1) function on the brain kinetics of metoclopramide. J Nucl Med 57:309–314. https://doi.org/10.2967/jnumed.115.164350
Innis RB, Cunningham VJ, Delforge J et al (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. https://doi.org/10.1038/sj.jcbfm.9600493
Auvity S, Caillé F, Marie S et al (2018) P-Glycoprotein (ABCB1) inhibits the influx and increases the efflux of 11C-metoclopramide across the blood-brain barrier: a PET study on nonhuman primates. J Nucl Med 59:1609–1615. https://doi.org/10.2967/jnumed.118.210104
Breuil L, Ziani N, Leterrier S et al (2022) Impact of cytochrome induction or inhibition on the plasma and brain kinetics of [11C]metoclopramide, a PET probe for p-glycoprotein function at the blood-brain barrier. Pharmaceutics 14:2650. https://doi.org/10.3390/pharmaceutics14122650
Breuil L, Marie S, Goutal S et al (2022) Comparative vulnerability of PET radioligands to partial inhibition of P-glycoprotein at the blood-brain barrier: a criterion of choice? J Cereb Blood Flow Metab 42:175–185. https://doi.org/10.1177/0271678X211045444
Bauer M, Karch R, Zeitlinger M et al (2015) Approaching complete inhibition of P-glycoprotein at the human blood-brain barrier: an (R)-[11C]verapamil PET study. J Cereb Blood Flow Metab 35:743–746. https://doi.org/10.1038/jcbfm.2015.19
Taki J, Sumiya H, Asada N et al (1998) Assessment of P-glycoprotein in patients with malignant bone and soft-tissue tumors using technetium-99m-MIBI scintigraphy. J Nucl Med 39:1179–1184
Feldmann M, Asselin M-C, Liu J et al (2013) P-glycoprotein expression and function in patients with temporal lobe epilepsy: a case-control study. Lancet Neurol 12:777–785. https://doi.org/10.1016/S1474-4422(13)70109-1
Funding
Funding by ANR19-CE17-0027 (EPIFLUX). LB AND DV received a joined grant from CEA/AP-HP.
Author information
Authors and Affiliations
Contributions
NT, LB, MB, and OL designed the study. LB, ME, DV, SM, SA, MB, SG, and SR analyzed the data. LB and NT wrote the first draft of the manuscript. All authors provided critical review and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Breuil, L., El Biali, M., Vodovar, D. et al. Parametric Imaging of P-Glycoprotein Function at the Blood–Brain Barrier Using kE,brain-maps Generated from [11C]Metoclopramide PET Data in Rats, Nonhuman Primates and Humans. Mol Imaging Biol 25, 1135–1141 (2023). https://doi.org/10.1007/s11307-023-01864-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01864-z