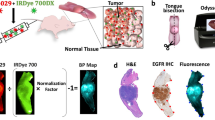
Abstract
Purpose
In nonmetastatic head and neck cancer treatment, surgical margin status is the most important prognosticator of recurrence and patient survival. Fresh frozen sectioning (FFS) of tissue margins is the standard of care for intraoperative margin assessment. However, FFS is time intensive, and its accuracy is not consistent among institutes. Mapping the epidermal growth factor receptor (EGFR) using paired-agent imaging (PAI) has the potential to provide more consistent intraoperative margin assessment in a fraction of the time as FFS.
Procedures
PAI was carried out through IV injection of an anti-epidermal growth factor receptor (EGFR) affibody molecule (ABY-029, eIND 122,681) and an untargeted IRDye680LT carboxylate. Imaging was performed on 4 µm frozen sections from three oral squamous cell carcinoma xenograft mouse models (n = 24, 8 samples per cell line). The diagnostic ability and tumor contrast were compared between binding potential, targeted, and untargeted images. Confidence maps were constructed based on group histogram-derived tumor probability curves. Tumor differentiability and contrast by confidence maps were evaluated.
Results
PAI outperformed ABY-029 and IRDye 680LT alone, demonstrating the highest individual receiver operating characteristic (ROC) curve area under the curve (PAI AUC: 0.91, 0.90, and 0.79) and contrast-to-noise ratio (PAI CNR: 1, 1.1, and 0.6) for FaDu, Det 562, and A253. PAI confidence maps (PAI CM) maintain high tumor diagnostic ability (PAI CMAUC: 0.91, 0.90, and 0.79) while significantly enhancing tumor contrast (PAI CMCNR: 1.5, 1.3, and 0.8) in FaDu, Det 562, and A253. Additionally, the PAI confidence map allows avascular A253 to be differentiated from a healthy tissue with significantly higher contrast than PAI. Notably, PAI does not require additional staining and therefore significantly reduces the tumor delineation time in a 5 \(\times\) 5 mm slice from ~ 35 min to under a minute.
Conclusion
This study demonstrated that PAI improved tumor detection in frozen sections with high diagnostic accuracy and rapid analysis times. The novel PAI confidence map improved the contrast in vascular tumors and differentiability in avascular tumors. With a larger database, the PAI confidence map promises to standardize fluorescence imaging in intraoperative pathology-assisted surgery (IPAS).






Similar content being viewed by others
References
Loree TR, Strong EW (1990) Significance of positive margins in oral cavity squamous carcinoma. Am J Surg 160:410–414. https://doi.org/10.1016/s0002-9610(05)80555-0
Eldeeb H, Macmillan C, Elwell C, Hammod A (2012) The Effect of the surgical margins on the outcome of patients with head and neck squamous cell carcinoma: single institution experience. Cancer Biol Med 9:29–33
Binahmed A, Nason RW, Abdoh AA (2007) The clinical significance of the positive surgical margin in oral cancer. Oral Oncol 43:780–784
Woolgar JA, Triantafyllou A (2005) A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol 41:1034–1043. https://doi.org/10.1016/j.oraloncology.2005.06.008
Meier JD, Oliver DA, Varvares MA (2005) Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck 27:952–958. https://doi.org/10.1002/hed.20269
Nayanar SK, Krishnan M K, K I M et al (2019) Frozen section evaluation in head and neck oncosurgery: an initial experience in a tertiary cancer center. Turk Patoloji Derg 35:46–51.https://doi.org/10.5146/tjpath.2018.01439
Du E, Ow TJ, Lo Y-T et al (2016) Refining the utility and role of frozen section in head and neck squamous cell carcinoma resection. Laryngoscope 126:1768–1775. https://doi.org/10.1002/lary.25899
Ord RA, Aisner S (1997) Accuracy of frozen sections in assessing margins in oral cancer resection. J Oral Maxillofac Surg 55:663–669; discussion 669–671. https://doi.org/10.1016/s0278-2391(97)90570-x
Bilodeau EA, Chiosea S (2011) Oral squamous cell carcinoma with mandibular bone invasion: intraoperative evaluation of bone margins by routine frozen section. Head Neck Pathol 5:216–220. https://doi.org/10.1007/s12105-011-0264-0
Li MM, Puram SV, Silverman DA et al (2019) Margin analysis in head and neck cancer: state of the art and future directions. Ann Surg Oncol 26:4070–4080. https://doi.org/10.1245/s10434-019-07645-9
Voskuil FJ, Vonk J, van der Vegt B et al (2021) Intraoperative imaging in pathology-assisted surgery. Nat Biomed Eng 6:503–514. https://doi.org/10.1038/s41551-021-00808-8
Brouwer de Koning SG, Weijtmans P, Karakullukcu MB et al (2020) Toward assessment of resection margins using hyperspectral diffuse reflection imaging (400–1,700 nm) during tongue cancer surgery. Lasers Surg Med 52:496–502. https://doi.org/10.1002/lsm.23161
Ji M, Lewis S, Camelo-Piragua S et al (2015) Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci Transl Med 7(309):309ra163 1–12. https://doi.org/10.1126/scitranslmed.aab0195
Glaser AK, Reder NP, Chen Y et al (2017) Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat Biomed Eng 1:0084. https://doi.org/10.1038/s41551-017-0084
Rosenthal EL, Moore LS, Tipirneni K et al (2017) Sensitivity and specificity of cetuximab-IRDye800CW to identify regional metastatic disease in head and neck cancer. Clin Cancer Res 23:4744–4752
Zhou Q, van den Berg NS, Rosenthal EL et al (2021) EGFR-targeted intraoperative fluorescence imaging detects high-grade glioma with panitumumab-IRDye800 in a phase 1 clinical trial. Theranostics 11:7130–7143. https://doi.org/10.7150/thno.60582
de Jongh SJ, Tjalma JJJ, Koller M et al (2020) Back-table fluorescence-guided imaging for circumferential resection margin evaluation using bevacizumab-800CW in patients with locally advanced rectal cancer. J Nucl Med 61:655–661. https://doi.org/10.2967/jnumed.119.232355
Rosenthal EL, Kulbersh BD, Duncan RD et al (2006) In vivo detection of head and neck cancer orthotopic xenografts by immunofluorescence. Laryngoscope 116:1636–1641
Wang C, Xu X, Folaron M et al (2021) Improved discrimination of tumors with low and heterogeneous EGFR expression in fluorescence-guided surgery through paired-agent protocols. Mol Imaging Biol. https://doi.org/10.1007/s11307-021-01656-3
Wang C, Xu X, Hodge S et al (2021) Identification of a suitable untargeted agent for the clinical translation of ABY-029 paired-agent imaging in fluorescence-guided surgery. Mol Imaging Biol. https://doi.org/10.1007/s11307-021-01642-9
Samkoe KS, Hull S, Elliott J et al (2020) Perspectives on the phase 0 clinical trial of microdose administration of ABY-029 for fluorescence guided surgery: stability testing. Proceedings of the SPIE: molecular-guided surgery: molecules, devices, and applications VI, vol 112220X. https://doi.org/10.1117/12.2547110
Fasano M, Della Corte CM, Viscardi G et al (2021) Head and neck cancer: the role of anti-EGFR agents in the era of immunotherapy. Ther Adv Med Oncol 13:1758835920949418. https://doi.org/10.1177/1758835920949418
Samkoe KS, Gunn JR, Marra K et al (2017) Toxicity and pharmacokinetic profile for single-dose injection of ABY-029: a fluorescent anti-EGFR synthetic affibody molecule for human use. Mol Imag Biol 19:512–521
Bhattacharya A, Tóth K, Mazurchuk R et al (2004) Lack of microvessels in well-differentiated regions of human head and neck squamous cell carcinoma A253 associated with functional magnetic resonance imaging detectable hypoxia, limited drug delivery, and resistance to irinotecan therapy. Clin Cancer Res 10:8005–8017. https://doi.org/10.1158/1078-0432.CCR-04-1306
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661. https://doi.org/10.1096/fj.07-9574LSF
Tichauer KM, Samkoe KS, Sexton KJ et al (2012) Improved tumor contrast achieved by single time point dual-reporter fluorescence imaging. J Biomed Opt 17:066001. https://doi.org/10.1117/1.JBO.17.6.066001
Abbas SA, Ikram M, Tariq MU et al (2017) Accuracy of frozen sections in oral cancer resections, an experience of a tertiary care hospital. J Pak Med Assoc 67:806–809
Cardiff RD, Miller CH, Munn RJ (2014) Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Prot pp 655–658. https://doi.org/10.1101/pdb.prot073411
Black C, Marotti J, Zarovnaya E, Paydarfar J (2006) Critical evaluation of frozen section margins in head and neck cancer resections. Cancer 107:2792–2800. https://doi.org/10.1002/cncr.22347
Gandour-Edwards RF, Donald PJ, Wiese DA (1993) Accuracy of intraoperative frozen section diagnosis in head and neck surgery: experience at a university medical center. Head Neck 15:33–38. https://doi.org/10.1002/hed.2880150108
Byers RM, Bland KI, Borlase B, Luna M (1978) The prognostic and therapeutic value of frozen section determinations in the surgical treatment of squamous carcinoma of the head and neck. Am J Surg 136:525–528. https://doi.org/10.1016/0002-9610(78)90275-1
Schmidt RL, Hunt JP, Hall BJ et al (2011) A systematic review and meta-analysis of the diagnostic accuracy of frozen section for parotid gland lesions. Am J Clin Pathol 136:729–738. https://doi.org/10.1309/AJCP2SD8RFQEUZJW
Frangioni JV (2003) In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7:626–634. https://doi.org/10.1016/j.cbpa.2003.08.007
Olson MT, Ly QP, Mohs AM (2019) Fluorescence guidance in surgical oncology: challenges, opportunities, and translation. Mol Imaging Biol 21:200–218. https://doi.org/10.1007/s11307-018-1239-2
Amit M, Na’ara S, Leider-Trejo L et al (2016) Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: a prospective randomized controlled study. Head Neck 38(Suppl 1):E1803-1809. https://doi.org/10.1002/hed.24320
Lacroix M, Toms SA (2014) Maximum safe resection of glioblastoma multiforme. JCO 32:727–728. https://doi.org/10.1200/JCO.2013.53.2788
King DM, Hackbarth DA, Kirkpatrick A (2012) Extremity soft tissue sarcoma resections: how wide do you need to be? Clin Orthop Relat Res 470:692–699. https://doi.org/10.1007/s11999-011-2167-5
Acknowledgements
The authors would like to acknowledge the gift of ABY-029 (R01 CA167413). The IRDye 680LT NHS ester was graciously provided by LI-COR Biosciences, Inc. as part of an ongoing academic-industrial partnership. The authors thank Pathology Translational Research Program at Dartmouth-Hitchcock Medical Center and histotechnologist Scott M. Palisoul ASCP (HT), who performed the frozen sectioning, IHC, and H&E staining.
Funding
This work was funded by the grant R37 CA212187 (KSS). The production of ABY-029 was funded by R01 CA167413.
Author information
Authors and Affiliations
Contributions
CW designed the research, performed the experiments, analyzed the data, and wrote the manuscript. SH, DR, and PJH helped perform the experiments, analyzed the data, and/or interpreted the data. EYC and KMT helped design the study and interpreted the data. KSS designed the research, provided the funding, interpreted the data, and supervised the experiments. All the authors read, edited, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Author KSS reports receiving the Odyssey M imaging system from LI-COR Biosciences, Inc. as a gift. All the other authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Hodge, S., Ravi, D. et al. Rapid and Quantitative Intraoperative Pathology-Assisted Surgery by Paired-Agent Imaging-Derived Confidence Map. Mol Imaging Biol 25, 190–202 (2023). https://doi.org/10.1007/s11307-022-01780-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01780-8