Abstract
Purpose
Previous studies indicate that 99mTc- and fluorescent-labeled c[Cys-Thr-Pro-Ser-Pro-Phe-Ser-His-Cys]OH (TCP-1) peptides were able to detect colorectal cancer (CRC) and tumor-associated vasculature. This study was designed to characterize the targeting properties of PEGylated and non-PEGylated TCP-1 peptides for CRC imaging.
Procedures
Cell uptake of cyanine 7 (Cy7)-labeled TCP-1 probes (Cy7-PEG4-TCP-1 and Cy7-TCP-1) was investigated in three CRC cell lines (human, HCT116 and HT29; mouse, CT26). Xenograft and orthotopic CRC tumor models with HCT116 and CT26 cells were used to characterize biodistribution and CRC tumor-targeting properties of TCP-1 fluorescence and radioligand with and without PEGylation, [99mTc]Tc-HYNIC-PEG4-TCP-1 vs. [99mTc]Tc-HYNIC-TCP-1.
Results
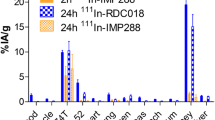
Fluorescence images showed that TCP-1 probes were distributed in the cytoplasm and nucleus of CRC cells. When CT26 cells were treated with unlabeled TCP-1 peptide prior to the cell incubation with Cy7-PEG4-TCP-1, cell fluorescent signals were significantly reduced relative to the cells without blockade. Relative to Cy7-TCP-1, superior brilliance and visibility of fluorescence was observed in the tumor with Cy7-PEG4-TCP-1 and maintained up to 18 h post-injection. [99mTc]Tc-HYNIC-PEG4-TCP-1 images in xenograft and orthotopic CRC models demonstrated that TCP-1 PEGylation preserved tumor-targeting capability of TCP-1, but its distribution (%ID/g) in the liver and intestine was higher than that of [99mTc]Tc-HYNIC-TCP-1 (1.51 ± 0.29 vs 0.53 ± 0.12, P < 0.01). Better tumor visualization by [99mTc]Tc-HYNIC-TCP-1 was observed in the orthotopic CRC model due to lower intestinal radioactivity.
Conclusions
TCP-1-based probes undergo endocytosis and localize in the cytoplasm and nucleus of human and mouse CRC cells. Tumor detectability of fluorescent TCP-1 peptide with a PEG4 spacer is promising due to its enhanced tumor binding affinity and rapid clearance kinetics from nontumor tissues. Non-PEGylated [99mTc]Tc-HYNIC-TCP-1 exhibits lower nonspecific accumulation in the liver and gastrointestinal tract and might have better capability for detecting CRC lesions in clinical sites. TCP-1 may represent an innovative targeting molecule for detecting CRC noninvasively.







Similar content being viewed by others
References
Li ZJ, Cho CH (2012) Peptides as targeting probes against tumor vasculature for diagnosis and drug delivery. J Transl Med 10(Suppl 1):S1
Li ZJ, Wu WK, Ng SS et al (2010) A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J Control Release 148(3):292–302
Lu L, Li ZJ, Li LF et al (2015) Vascular-targeted TNFalpha improves tumor blood vessel function and enhances antitumor immunity and chemotherapy in colorectal cancer. J Control Release 210:134–146
Shen J, Li ZJ, Li LF et al (2016) Vascular-targeted TNFalpha and IFNgamma inhibits orthotopic colorectal tumor growth. J Transl Med 14(1):187
Liu Z, Gray BD, Barber C, Bernas M, Cai M et al (2016) Characterization of TCP-1 probes for molecular imaging of colon cancer. J Control Release 239:223–230
Yang ZH, Dang YQ, Ji G (2019) Role of epigenetics in transformation of inflammation into colorectal cancer. World J Gastroenterol 25(23):2863–2877
Cao Q, Liu S, Niu G, Chen K, Yan Y, Liu Z, Chen X (2011) Phage display peptide probes for imaging early response to bevacizumab treatment. Amino Acids 41(5):1103–1112
Enback J, Laakkonen P (2007) Tumour-homing peptides: tools for targeting, imaging and destruction. Biochem Soc Trans 35(Pt 4):780–783
Laakkonen P, Vuorinen K (2010) Homing peptides as targeted delivery vehicles. Integr Biol (Camb) 2(7–8):326–337
Laakkonen P, Zhang L, Ruoslahti E (2008) Peptide targeting of tumor lymph vessels. Ann N Y Acad Sci 1131:37–43
Sturzu A, Regenbogen M, Klose U et al (2008) Novel dual labelled nucleus-directed conjugates containing correct and mutant nuclear localisation sequences. Eur J Pharm Sci 33(3):207–216
Kalderon D, Roberts BL, Richardson WD, Smith AE (1984) A short amino acid sequence able to specify nuclear location. Cell 39(3 Pt 2):499–509
Erfani M, Zarrabi Ahrabi N, Shafiei M, Shirmardi SP (2014) A (99m) Tc-tricine-HYNIC-labeled peptide targeting the neurotensin receptor for single-photon imaging in malignant tumors. J Labelled Comp Radiopharm 57(3):125–131
Liao HW, Hung MC (2017) Intracaecal orthotopic colorectal cancer xenograft mouse model. Bio Protoc 7(11):e2311
Miller BW, Gregory SJ, Fuller ES et al (2014) The iQID camera: an ionizing-radiation quantum imaging detector. Nucl Instrum Methods Phys Res A 767:146–152
Furenlid LR, Barrett HH, Barber HB, et al (2014) Molecular imaging in the College of Optical Sciences - an overview of two decades of instrumentation development. Proc SPIE Int Soc Opt Eng 9186
Han L, Miller BW, Barber HB, Nagarkar VV, Furenlid LR (2014) A new columnar CsI(Tl) scintillator for iQID detectors. Proc SPIE Int Soc Opt Eng 9214:92140D
Schottelius M, Wester HJ (2009) Molecular imaging targeting peptide receptors. Methods 48(2):161–177
Turker NS, Heidari P, Kucherlapati R, Kucherlapati M, Mahmood U (2014) An EGFR targeted PET imaging probe for the detection of colonic adenocarcinomas in the setting of colitis. Theranostics 4(9):893–903
Kim J, Do EJ, Moinova H et al (2017) Molecular imaging of colorectal tumors by targeting colon cancer secreted protein-2 (CCSP-2). Neoplasia 19(10):805–816
Oh P, Li Y, Yu J et al (2004) Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 429(6992):629–635
McGuire TF, Sajithlal GB, Lu J, Nicholls RD, Prochownik EV (2012) In vivo evolution of tumor-derived endothelial cells. PLoS One 7(5):e37138
Meseure D, Drak Alsibai K, Nicolas A (2014) Pivotal role of pervasive neoplastic and stromal cells reprogramming in circulating tumor cells dissemination and metastatic colonization. Cancer Microenviron 7(3):95–115
Turecek PL, Bossard MJ, Schoetens F, Ivens IA (2016) PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci 105(2):460–475
Li Q, White JB, Peterson NC et al (2018) Tumor uptake of pegylated diabodies: Balancing systemic clearance and vascular transport. J Control Release 279:126–135
Acknowledgements
The authors are grateful to Dr. Harrison Barrett, Director of the Center for Gamma-Ray Imaging, for making the facilities of the Center available for animal imaging studies. We wish to thank Dr. Gail Stevenson for support in animal care. We are grateful to Drs. Arthur Gmitro and Andrew Rouse for their assistance in cell fluorescence imaging.
Funding
This study was funded by NIH grants NCI 1R21-CA216657, NIBIB P41-EB002035, and NCI P30-CA023074.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. Animal protocols for cancer implantation and imaging studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Arizona.
Conflict of Interest
Drs. Koon Pak and Brian Gray are president and vice president for research of Molecular Targeting Technologies, Inc., respectively. Other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Z., Gray, B.D., Barber, C. et al. PEGylated and Non-PEGylated TCP-1 Probes for Imaging of Colorectal Cancer. Mol Imaging Biol 25, 133–143 (2023). https://doi.org/10.1007/s11307-021-01684-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01684-z