Abstract
Purpose
Chemical exchange saturation transfer MRI using an infusion of glucose (glucoCEST) is sensitive to the distribution of glucose in vivo; however, whether glucoCEST is more related to perfusion or glycolysis is still debatable. We compared glucoCEST to computed tomography perfusion (CTP), [18F] fluorodeoxyglucose positron emission tomography (FDG-PET), and hyperpolarized [1-13C] pyruvate magnetic resonance spectroscopy imaging (MRSI) in a C6 rat model of glioma to determine if glucoCEST is more strongly correlated with measurements of perfusion or glycolysis.
Methods
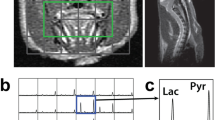
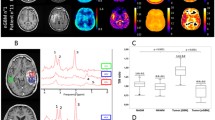
106 C6 glioma cells were implanted in Wistar rat brains (n = 11). CTP (including blood volume, BV; blood flow, BF; and permeability surface area product, PS) and FDG-PET standardized uptake value (SUV) were acquired at 11 to 13 days post-surgery. GlucoCEST measurements (∆CEST) were acquired the following day on a 9.4 T MRI before and after an infusion of glucose solution. This was followed by MRSI on a 3.0 T MRI after the injection of hyperpolarized [1-13C] pyruvate to generate regional maps of the lactate:pyruvate ratio (Lac:Pyr). Pearson’s correlations between glucoCEST, CTP, FDG-PET, and Lac:Pyr ratio were evaluated.
Results
Tumors had significantly higher SUV, BV, and PS than the contralateral brain. Tumor ∆CEST was most strongly correlated with CTP measurements of BV (ρ = 0.74, P = 0.01) and PS (ρ = 0.55, P = 0.04). No significant correlation was found between glycolysis measurements of SUV or Lac:Pyr with tumor ∆CEST. PS significantly correlated with SUV (ρ = 0.58, P = 0.005) and Lac:Pyr (ρ = 0.75, P = 0.005). BV significantly correlated with Lac:Pyr (ρ = 0.57, P = 0.02), and BF significantly correlated with SUV (ρ = 0.49, P = 0.02).
Conclusion
This study determined that glucoCEST is more strongly correlated to measurements of perfusion than glycolysis. GlucoCEST measurements have additional confounds, such as sensitivity to changing pH, that merit additional investigation.






Similar content being viewed by others
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumors. Nat Rev Neurosci 8(8):610–622
Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA, Mickey BE, Pan E, Sherry AD, Bachoo RM, Takahashi M (2014) In vivo chemical exchange saturation imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci U S A 111(12):4542–4547
Chan KW, McMahon MT, Kato Y et al (2012) Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med 68(6):1764–1773
Torrealdea F (2016) Investigation of brain tumor metabolism using naturally occurring chemical exchange saturation transfer agents with magnetic resonance imaging. PhD Thesis, University College London
Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Gonçalves M, Parkes HG, Årstad E, Thomas DL, Pedley RB, Lythgoe MF, Golay X (2013) In vivo imaging of glucose uptake and metabolism in tumors. Nat Med 19(8):1067–1072
Jin T, Mehrens H, Hendrick KS, Kim SG (2014) Mapping glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J Cerebral Metab 34:1402–1410
Xu X, Yadav N, Knutsson L et al (2015) Dynamic glucose-enhance (DGE) MRI: translation to human scanning and first results in glioma patients. Tomography 1(2):105–114
Jin T, Lordanova B, Hitchens TK et al (2018) Chemical exchange-sensitive spin-lock (CESL) MRI of glucose and analogs in brain tumors. MR Med 80:488–495
Qi Q, Yeung TPC, Lee TY, Bauman G, Crukley C, Morrison L, Hoffman L, Yartsev S (2016) Evaluation of CT perfusion biomarkers of tumor hypoxia. PLoS One 11(4):e0153569
Yeung TPC, Wang Y, He W et al (2015) Survival prediction in high-grade gliomas using CT perfusion imaging. J Neuro-Oncol 123:93–123
Yeung TPC, Bauman G, Yartsev S, Fainardi E, Macdonald D, Lee TY (2015) Dynamic perfusion CT in brain tumors. Eur J Radiol 84(12):2386–2392
Lee TY, Purdie TG, Stewart E (2003) CT imaging of angiogenesis. QJ Nucl Med 41:171–187
Tahari AK, Chien D, Azadi J et al (2014) Optimum lean body formulation for correction of standardized uptake value in PET imaging. JNM 55:1481–1484
Liu G, Song X, Chan KW et al (2013) Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed 26(7):810–828
Lim, H (2017) A Longitudinal study of tumor metabolism using hyperpolarized carbon-13 magnetic resonance spectroscopic imaging in a preclinical model of glioma. PhD Thesis, Western University, London
Bonavia R, Inda M, Cavanee WK et al (2011) Heterogeneity maintenance in glioblastoma: a social network. Cancer Res 71:4055–4060
Mehrabian H, Desmon KL, Soliman H et al (2017) Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res 23:3667–3675
Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl P (2010) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17(1):130–134
Hogeboom WR, Hoekstra HJ, Mooyaart EI et al (1991) MRI and CT in the preoperative evaluation of soft-tisue tumors. Arch Orthop Trauma Surg 110:162–164
Xu X, Chan K, Knutsson L et al (2015) Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. Magn Reason Med 74:1556–1563
Cao Y, Nagesh V, Hasmstra D et al (2006) The extent and severity of vascular leakage as evidence of tumor aggressiveness in high-grade gliomas. Cancer Res 66(17):8912–8917
Hundershammer C, Braeuer M, Müller CA et al (2018) Simultaneous characterization of tumor cellularity and the Warburg effect with PET, MRI and hyperpolarized 13C-MRSI. Theranostics 8(17):4765–4780
Twarock S, Reichert C, Peters U, Gorski DJ, Röck K, Fischer JW (2017) Hyperglycemia and aberrated insulin signaling stimulate tumor progression via induction of extracellular matrix component hyaluronan. Int J Cancer 141:791–804
Lund J, Ouwens DM, Wettergreen M et al (2019) Increased glycolysis and higher lactate production in hyperglycemic myotubes. Cells 8(9):1101. https://doi.org/10.3390/cells8091101
Hsu T, Nguyen-Tran HH, Trojanowska M (2019) Active roles of dysfunctional vascular endothelium in fibrosis and cancer. J Biomed Sci 26:86
Blystad I, Warntjes JBM, Smedby Ö, Lundberg P, Larsson EM, Tisell A (2017) Quantitative MRI for analysis of peritumoural edema in malignant gliomas. PLoS ONE 12(5):e0177135. https://doi.org/10.1371/journal.pone.0177135
Furger BJ, Czernin J, Hildebrandt, et al. (2006) Impact of animal handling on the results of 18F-FDG PET study in mice. J Nucl Med 47(6):999–1006
Kim D, Ko HY, Lee S, Lee YH, Ryu S, Kim SY, Chung JI, Lee M, Moon JH, Chang JH, Yun M (2020) Glucose loading enhances the value of 18F-FDG PET/CT for the characterization and delineation of cerebral gliomas. Cancers 12(7). https://doi.org/10.3390/cancers12071977
Jensen TL, Kiersgaard MK, Sørensen DB, Mikkelsen LF (2013) Fasting of mice: a review. Lab Anim 47(4):225–240
Sarikaya I, Albatineh AN, Sarikayaa A (2020) Effect of various blood glucose levels on regional FDG uptake in the brain. Asia Ocean J Nucl Med Biol 8(1):46–53
Todd MM, Weeks JB, Warner DS (1993) The influence of intravascular volume expansion on cerebral blood flow and blood volume in normal rats. Anesthesiology 78:945–953
Cole DJ, Drummond JC, Mastumura JS et al (1990) Hypervolemic-hemodilution and hypertensive during temporary middle cerebral artery occlusion in rats: the effect on blood-brain barrier permeability. Can J Neurol Sci 17:372–377
Christiansen JS, Frandsen M, Parving HH (1981) Effect of intravenous glucose infusion on renal function in normal man and insulin-dependent diabetics. Diabetologia 21:368–373
van Zijl PCM, Yadav NN (2011) Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn Reson Med 65(4):927–948
Sun PZ, Sorensen AG (2008) Imaging pH using the chemical exchange saturation transfer (CEST) MRI: Correction of concomitant RF irradiation effects to quantify CEST MRI for chemical exchange rate and pH. Magn Reson Med 60(2):390–397
Roszinski S, Wiedemann G, Jiang SZ, Baretton G, Wagner T, Weiss C (1991) Effects of hyperthermia and/or hyperglycemia on pH and pO2 in well oxygenated xenotransplanted human sarcoma. Inl J Radiat Oncol Biol Phys 20(6):1273–1280
Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD (2014) Evaluation of extracellular pH within in vivo tumors using acidoCEST MRI. Magn Reson Med 72(5):1408–1417
Longo DL, Bartoli A, Consolino L, Bardini P, Arena F, Schwaiger M, Aime S (2016) In vivo imaging of tumor metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res 76(22):6463–6470
McVicar N, Li A, Gonҫalves DF et al (2014) Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab 34(4):690–698
Xu X, Xu J, Knutsson L, el al. (2019) The effect of the mTOR inhibitor rapamycin on glucoCEST singal in a preclinical model of glioblastoma. Magn Reson Med 81(6):3798–3807
Nasrallah FA, Pages G, Kuchel PW et al (2013) Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J Cereb Blood Flow Metab 33:1270–1278
Sehgal AA, Li Y, Lal B et al (2018) CEST MRI of 3-O-methyl-D-glucose and accumulation in brain tumors. Magn Reson Med 81:1993–2000
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest for this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure 1
Average tumor size on first imaging day for PET and CT perfusion experiment and the next day for glucoCEST and hyperpolarized [1-13C]pyruvate. No statistical significance in tumor size were found between these two subsequent imaging days (P = 0.54). Error bars = standard deviation. (PNG 75 kb)
Supplementary Figure 2
An illustrative example of blood glucose change during the 60-min constant infusion. Time 0 was defined as the time at the start of constant infusion of 20 % glucose solution (1.5 g/kg) and after a bolus of 20 % glucose solution (0.3 g/kg) was injected. (PNG 26 kb)
Supplementary Figure 3
Mean values from ROIs defined in both tumor and contralateral brain tissue in AUCMTR pre- and During infusion (30-60 min). A statistically significant (P < 0.05) difference was found between tumor and contralateral side in AUCMTR pre- and during glucose infusion. (DOCX 20 kb)
ESM 1
(PNG 28 kb)
Rights and permissions
About this article
Cite this article
Qi, Q., Fox, M.S., Lim, H. et al. Multimodality In Vivo Imaging of Perfusion and Glycolysis in a Rat Model of C6 Glioma. Mol Imaging Biol 23, 516–526 (2021). https://doi.org/10.1007/s11307-021-01585-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01585-1