Abstract
Purpose
Differentiation between radiation-induced necrosis and tumor recurrence is crucial to determine proper management strategies but continues to be one of the central challenges in neuro-oncology. We hypothesized that hyperpolarized 13C MRI, a unique technique to measure real-time in vivo metabolism, would distinguish radiation necrosis from tumor on the basis of cell-intrinsic metabolic differences. The purpose of this study was to explore the feasibility of using hyperpolarized [1-13C]pyruvate for differentiating radiation necrosis from brain tumors.
Procedures
Radiation necrosis was initiated by employing a CT-guided 80-Gy single-dose irradiation of a half cerebrum in mice (n = 7). Intracerebral tumor was modeled with two orthotopic mouse models: GL261 glioma (n = 6) and Lewis lung carcinoma (LLC) metastasis (n = 7). 13C 3D MR spectroscopic imaging data were acquired following hyperpolarized [1-13C]pyruvate injection approximately 89 and 14 days after treatment for irradiated and tumor-bearing mice, respectively. The ratio of lactate to pyruvate (Lac/Pyr), normalized lactate, and pyruvate in contrast-enhancing lesion was compared between the radiation-induced necrosis and brain tumors. Histopathological analysis was performed from resected brains.
Results
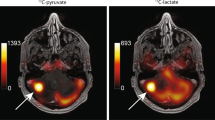
Conventional MRI exhibited typical radiographic features of radiation necrosis and brain tumor with large areas of contrast enhancement and T2 hyperintensity in all animals. Normalized lactate in radiation necrosis (0.10) was significantly lower than that in glioma (0.26, P = .004) and LLC metastatic tissue (0.25, P = .00007). Similarly, Lac/Pyr in radiation necrosis (0.18) was significantly lower than that in glioma (0.55, P = .00008) and LLC metastasis (0.46, P = .000008). These results were consistent with histological findings where tumor-bearing brains were highly cellular, while irradiated brains exhibited pathological markers consistent with reparative changes from radiation necrosis.
Conclusion
Hyperpolarized 13C MR metabolic imaging of pyruvate is a noninvasive imaging method that differentiates between radiation necrosis and brain tumors, providing a groundwork for further clinical investigation and translation for the improved management of patients with brain tumors.





Similar content being viewed by others
References
Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ (2017) Response assessment in neuro-oncology clinical trials. J Clin Oncol 35:2439–2449
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro-Oncology 15:515–534
Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH et al (2019) Hyperpolarized (13)C MRI: path to clinical translation in oncology. Neoplasia (New York, NY) 21:1–16
Wang ZJ, Ohliger MA, Larson PEZ, Gordon JW, Bok RA, Slater J, Villanueva-Meyer JE, Hess CP, Kurhanewicz J, Vigneron DB (2019) Hyperpolarized (13)C MRI: state of the art and future directions. Radiology 291:273–284
Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K (2003) Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 100:10158–10163
Autry AW, Hashizume R, James CD, Larson PEZ, Vigneron DB, Park I (2018) Measuring tumor metabolism in pediatric diffuse intrinsic pontine glioma using hyperpolarized carbon-13 MR metabolic imaging. Contrast Media Mol Imaging 2018:3215658
Park I, Larson PE, Zierhut ML et al (2010) Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro-Oncology 12:133–144
Day SE, Kettunen MI, Cherukuri MK, Mitchell JB, Lizak MJ, Morris HD, Matsumoto S, Koretsky AP, Brindle KM (2011) Detecting response of rat C6 glioma tumors to radiotherapy using hyperpolarized [1- 13C]pyruvate and 13C magnetic resonance spectroscopic imaging. Magn Reson Med 65:557–563
Michel KA, Zielinski R, Walker CM et al (2019) Hyperpolarized pyruvate MR spectroscopy depicts glycolytic inhibition in a mouse model of glioma. Radiology 293:168–173
Park I, Bok R, Ozawa T, Phillips JJ, James CD, Vigneron DB, Ronen SM, Nelson SJ (2011) Detection of early response to temozolomide treatment in brain tumors using hyperpolarized 13C MR metabolic imaging. J Magn Reson Imaging 33:1284–1290
Park I, Mukherjee J, Ito M, Chaumeil MM, Jalbert LE, Gaensler K, Ronen SM, Nelson SJ, Pieper RO (2014) Changes in pyruvate metabolism detected by magnetic resonance imaging are linked to DNA damage and serve as a sensor of temozolomide response in glioblastoma cells. Cancer Res 74:7115–7124
Park JM, Spielman DM, Josan S, Jang T, Merchant M, Hurd RE, Mayer D, Recht LD (2016) Hyperpolarized (13)C-lactate to (13)C-bicarbonate ratio as a biomarker for monitoring the acute response of anti-vascular endothelial growth factor (anti-VEGF) treatment. NMR Biomed 29:650–659
Park I, Hu S, Bok R, Ozawa T, Ito M, Mukherjee J, Phillips JJ, James CD, Pieper RO, Ronen SM, Vigneron DB, Nelson SJ (2013) Evaluation of heterogeneous metabolic profile in an orthotopic human glioblastoma xenograft model using compressed sensing hyperpolarized 3D 13C magnetic resonance spectroscopic imaging. Magn Reson Med 70:33–39
Park I, Lupo JM, Nelson SJ (2019) Correlation of tumor perfusion between carbon-13 imaging with hyperpolarized pyruvate and dynamic susceptibility contrast MRI in pre-clinical model of Glioblastoma. Mol Imaging Biol 21:626–632
Crane JC, Olson MP, Nelson SJ (2013) SIVIC: open-source, standards-based software for DICOM MR spectroscopy workflows. Int J Biomed Imaging 2013:169526
Warburg O (1956) On the origin of cancer cells. Science (New York, NY) 123:309–314
Nakamura JL, Verhey LJ, Smith V, Petti PL, Lamborn KR, Larson DA, Wara WM, McDermott MW, Sneed PK (2001) Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys 51:1313–1319
Sneed PK, Mendez J, Vemer-van den Hoek JG et al (2015) Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg 123:373–386
Münter MW, Karger CP, Reith W, Schneider HM, Peschke P, Debus J (1999) Delayed vascular injury after single high-dose irradiation in the rat brain: histologic immunohistochemical, and angiographic studies. Radiology 212:475–482
Niranjan A, Gobbel GT, Kondziolka D, Flickinger JC, Lunsford LD (2004) Experimental radiobiological investigations into radiosurgery: present understanding and future directions. Neurosurgery 55:495–504 discussion 504-495
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, McKeever PE, Chenevert TL (2006) Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24:1131–1142
Barajas RF Jr, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, Cha S (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496
Huang J, Wang AM, Shetty A, Maitz AH, Yan D, Doyle D, Richey K, Park S, Pieper DR, Chen PY, Grills IS (2011) Differentiation between intra-axial metastatic tumor progression and radiation injury following fractionated radiation therapy or stereotactic radiosurgery using MR spectroscopy, perfusion MR imaging or volume progression modeling. Magn Reson Imaging 29:993–1001
Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PCM (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17:130–134
Chen W, Silverman DH, Delaloye S et al (2006) 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47:904–911
Rachinger W, Goetz C, Popperl G et al (2005) Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57:505–511 discussion 505-511
Van Laere K, Ceyssens S, Van Calenbergh F et al (2005) Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging 32:39–51
Wilson DM, Keshari KR, Larson PE et al (2010) Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J Magn Reson (San Diego, Calif : 1997) 205:141–147
Hansen AE, Gutte H, Holst P, Johannesen HH, Rahbek S, Clemmensen AE, Larsen MME, Schøier C, Ardenkjaer-Larsen J, Klausen TL, Kristensen AT, Kjaer A (2018) Combined hyperpolarized (13)C-pyruvate MRS and (18)F-FDG PET (hyperPET) estimates of glycolysis in canine cancer patients. Eur J Radiol 103:6–12
Hundshammer C, Braeuer M, Müller CA, Hansen AE, Schillmaier M, Düwel S, Feuerecker B, Glaser SJ, Haase A, Weichert W, Steiger K, Cabello J, Schilling F, Hövener JB, Kjær A, Nekolla SG, Schwaiger M (2018) Simultaneous characterization of tumor cellularity and the Warburg effect with PET, MRI and hyperpolarized (13)C-MRSI. Theranostics 8:4765–4780
Witney TH, Kettunen MI, Day SE et al (2009) A comparison between radiolabeled fluorodeoxyglucose uptake and hyperpolarized (13)C-labeled pyruvate utilization as methods for detecting tumor response to treatment. Neoplasia (New York, NY) 11:574–582 571 p following 582
Hirschhaeuser F, Sattler UG, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71:6921–6925
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60:916–921
Brindle K (2008) New approaches for imaging tumour responses to treatment. Nat Rev Cancer 8:94–107
Kurhanewicz J, Vigneron DB, Brindle K et al (2011) Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia (New York, NY) 13:81–97
Kugel H, Heindel W, Ernestus RI, Bunke J, du Mesnil R, Friedmann G (1992) Human brain tumors: spectral patterns detected with localized H-1 MR spectroscopy. Radiology 183:701–709
Dodd MS, Ball V, Bray R, Ashrafian H, Watkins H, Clarke K, Tyler DJ (2013) In vivo mouse cardiac hyperpolarized magnetic resonance spectroscopy. J Cardiovasc Magn Reson 15:19
Chen Y, Kim H, Bok R, et al. Pyruvate to lactate metabolic changes during neurodevelopment measured dynamically using hyperpolarized 13C imaging in juvenile murine brain
Grist JT, McLean MA, Riemer F et al (2019) Quantifying normal human brain metabolism using hyperpolarized [1-(13)C]pyruvate and magnetic resonance imaging. NeuroImage 189:171–179
Miloushev VZ, Granlund KL, Boltyanskiy R, Lyashchenko SK, DeAngelis LM, Mellinghoff IK, Brennan CW, Tabar V, Yang TJ, Holodny AI, Sosa RE, Guo YWW, Chen AP, Tropp J, Robb F, Keshari KR (2018) Metabolic imaging of the human brain with hyperpolarized (13)C pyruvate demonstrates (13)C lactate production in brain tumor patients. Cancer Res 78:3755–3760
Park I, Larson PEZ, Gordon JW, Carvajal L, Chen HY, Bok R, van Criekinge M, Ferrone M, Slater JB, Xu D, Kurhanewicz J, Vigneron DB, Chang S, Nelson SJ (2018) Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med 80:864–873
Autry AW, Gordon JW, Chen HY, LaFontaine M, Bok R, van Criekinge M, Slater JB, Carvajal L, Villanueva-Meyer JE, Chang SM, Clarke JL, Lupo JM, Xu D, Larson PEZ, Vigneron DB, Li Y (2020) Characterization of serial hyperpolarized (13)C metabolic imaging in patients with glioma. NeuroImage Clin 27:102323
Acknowledgments
The authors would like to thank Chung-Man Moon, Biruk Tesfaye Birhanu, and Nguyen Trong Nguyen for the assistance with the experimental procedure.
Funding
This study was supported by the Ministry of Science and ICT, Republic of Korea (2017R1C1B5018396), the Ministry of Education, Republic of Korea (2019R1I1A3A01059201), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR20C0021), American Brain Tumor Association (DG1700010), and the DongKook Life Science. Co., Ltd., Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 116 kb)
Rights and permissions
About this article
Cite this article
Park, I., Kim, S., Pucciarelli, D. et al. Differentiating Radiation Necrosis from Brain Tumor Using Hyperpolarized Carbon-13 MR Metabolic Imaging. Mol Imaging Biol 23, 417–426 (2021). https://doi.org/10.1007/s11307-020-01574-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01574-w