Abstract
Purpose
Myocardial healing following myocardial infarction (MI) is a complex process that is yet to be fully understood. Clinical attempts in regeneration of the injured myocardium using cardiac stem cells faced major challenges, calling for a better understanding of the processes involved at a more basic level in order to foster translation.
Procedures
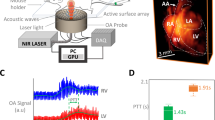
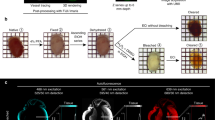
We examined the feasibility of volumetric optoacoustic tomography (VOT) in studying healing of the myocardium in different models of MI, including permanent occlusion (PO) of the left coronary artery, temporary occlusion (ischemia-reperfusion—I/R) and infarcted c-kit mutants, a genetic mouse model with impaired cardiac healing. Murine hearts were imaged at 100 Hz frame rate using 800 nm excitation wavelength, corresponding to the peak absorption of indocyanine green (ICG) in plasma and the isosbestic point of haemoglobin.
Results
The non-invasive real-time volumetric imaging capabilities of VOT have allowed the detection of significant variations in the pulmonary transit time (PTT), a parameter affected by MI, across different murine models. Upon intravenous injection of ICG, we were able to track alterations in cardiac perfusion in I/R models, which were absent in wild-type (wt) PO or kitW/kitW-v PO mice. The wt-PO and I/R models further exhibited irregularities in their cardiac cycles.
Conclusions
Clear differences in the PTT, ICG perfusion and cardiac cycle patterns were identified between the different models and days post MI. Overall, the results highlight the unique capacity of VOT for multi-parametric characterization of morphological and functional changes in murine models of MI.





Similar content being viewed by others
References
Tanai E, Frantz S (2011) Pathophysiology of heart failure. Compr Physiol 6(1):187–214
Sutton MGSJ, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101(25):2981–2988
Kikuchi K, Poss KD (2012) Cardiac regenerative capacity and mechanisms. Annual Rev Cell Dev Biol 28:719–741
Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P (2005) Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res 97(7):663–673
Bergmann O, Bhardwaj RD, Bernanrd S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Burchholz BA, Duird H, Jovinge S, Frisen J (2009) Evidence for cardiomyocyte renewal in humans. Science 324(5923):98–102
Madigan M, Atoui R (2018) Therapeutic use of stem cells for myocardial infarction. Bioengineering 5(2):28
Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ (2014) Cell therapy for cardiac repair—lessons from clinical trials. Nat Rev Cardiol 11(4):232–246
Tompkins BA, Balkan W, Winkler J, Gyöngyösi M, Goliasch G, Fernández-Avilés F, Hare JM (2018) Preclinical studies of stem cell therapy for heart disease. Circ Res 122(7):1006–1020
Yu X, Qian C, Chen DY, Dodd SJ, Koretsky AP (2014) Deciphering laminar-specific neural inputs with line-scanning fMRI. Nat Methods 11(1):55–58
Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M (2018) Guidelines for measuring cardiac physiology in mice. Am J Physiol-Heart C 314(4):H733–H752
Provost J, Papadacci C, Arango JE, Imbault M, Fink M, Gennisson JL, Tanter M, Pernot M (2014) 3D ultrafast ultrasound imaging in vivo. Phys Med Biol 59(19):L1–L13
Damen FW, Berman AG, Soepriatna AH, Ellis JM, Buttars SD, Aasa KL, Goergen CJ (2017) High-frequency 4-dimensional ultrasound (4DUS): a reliable method for assessing murine cardiac function. Tomography 3(4):180–187
Deán-Ben XL, Ford SJ, Razansky D (2015) High-frame rate four dimensional optoacoustic tomography enables visualization of cardiovascular dynamics and mouse heart perfusion. Sci Rep 5:10133
Lin H-CA, Dean-Ben XL, Ivankovic I, Kimm MA, Kosanke K, Haas H, Meier R, Lohofer F, Wildgruber M, Razansky D (2017) Characterization of cardiac dynamics in an acute myocardial infarction model by four-dimensional optoacoustic and magnetic resonance imaging. Theranostics 7(18):4470–4479
Ivankovic I, Deán-Ben XL, Lin HCA, Zhang Z, Trautz B, Petry A, Gorlach A, Razansky D (2019) Volumetric optoacoustic tomography enables non-invasive in vivo characterization of impaired heart function in hypoxic conditions. Sci Rep 9(1):8369
Marino F, Scalise M, Cianflone E, Mancuso T, Aquila I, Agosti V, Torella M, Paolino D, Mollace V, Nadal-Ginard B, Torella D (2019) Role of c-kit in myocardial regeneration and aging. Front Endocrinol 10
Wildgruber M, Bielicki I, Aichler M, Kosanke K, Feuchtinger A, Settles M, Onthank DC, Cesati RR, Robinson SP, Huber AM, Rummeny EJ, Walch AK, Botnar RM (2014) Assessment of myocardial infarction and postinfarction scar remodeling with an elastin-specific magnetic resonance agent. Circ. Cardiovasc Imaging 7(2):321–329
Di Siena S, Gimmelli R, Nori SL, Barbagallo F, Campolo F, Dolci S, Feigenbaum L, Lenzi A, Naro F, Cianflone E, Mancuso T, Torella D, Isidori AM, Pellegrini M (2016) Activated c-Kit receptor in the heart promotes cardiac repair and regeneration after injury. Cell Death Dis 7(7):e2317
Deán-Ben XL, Ozbek A, Razansky D (2013) Volumetric real-time tracking of peripheral human vasculature with GPU-accelerated three-dimensional optoacoustic tomography. IEEE Trans Med Imaging 32(11):2050–2055
Muthuramu I, Lox M, Jacobs F, De Geest B (2014) Permanent ligation of the left anterior descending coronary artery in mice: a model of post-myocardial infarction remodelling and heart failure. Jove-J Vis Exp 94
Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frandogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G (2018) Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol-Heart C 314(4):H812–H838
Ganame J, Messalli G, Dymarkowski S, Rademakers FE, Desmet W, Van de Werf F, Bogaert J (2009) Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J 30(12):1440–1449
Hsieh PC et al (2007) Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13(8):970–974
Granger DN, Kvietys PR (2017) Reperfusion therapy—What’s with the obstructed, leaky and broken capillaries? Pathophysiology 24(4):213–228
Vandoorne K, Addadi Y, Neeman M (2010) Visualizing vascular permeability and lymphatic drainage using labeled serum albumin. Angiogenesis 13(2):75–85
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo PA (2012) review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 940585
Sonin D, Papayan G, Pochkaeva E, Chefu S, Minasian S, Kurapeev D, Vaage J, Petrishchev N, Galagudza M (2017) In vivo visualization and ex vivo quantification of experimental myocardial infarction by indocyanine green fluorescence imaging. Biomed Opt Express 8(1):151–161
Zimetbaum PJ, Josephson ME (2003) Use of the electrocardiogram in acute myocardial infarction. New Engl J Med 348(10):933–940
Cohen M, Boiangiu C, Abidi M (2010) Therapy for ST-segment elevation myocardial infarction patients who present late or are ineligible for reperfusion therapy. J Am Coll of Cardiol 55(18):1895–1906
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD (2012) Third universal definition of myocardial infarction. Circulation 126(16):2020–2035
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ivankovic, I., Déan-Ben, X.L., Haas, H. et al. Volumetric Optoacoustic Tomography Differentiates Myocardial Remodelling. Mol Imaging Biol 22, 1235–1243 (2020). https://doi.org/10.1007/s11307-020-01498-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01498-5