Abstract
Purpose
This work aims to identify intratumoral habitats with distinct heterogeneity based on 2-deoxy-2-[18F]fluro-d-glucose positron emission tomography (PET)/X-ray computed tomography (CT) imaging, and to develop a subregional radiomics approach to predict progression-free survival (PFS) in patients with nasopharyngeal carcinoma (NPC).
Procedures
In total, 128 NPC patients (85 vs. 43 for primary vs. validation cohorts) who underwent pre-treatment PET/CT scan were enrolled retrospectively. Each tumor was partitioned into several phenotypically consistent subregions based on individual- and population-level clustering. For each subregion, 202 radiomics features were extracted to construct imaging biomarker for prognosis via Cox’s proportional hazard model combined with forward stepwise feature selection. Relevance of imaging biomarkers and clinicopathological factors were assessed by multivariate Cox regression analysis and Spearman’s correlation analysis. To investigate whether imaging biomarkers could provide complementary prognosis information beyond existing predictors, a scoring system was further developed for risk stratification and compared with AJCC staging system.
Results
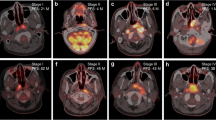
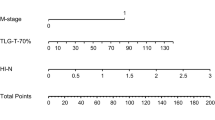
Three subregions (denoted as S1, S2, and S3) were discovered with distinct PET/CT imaging characteristics in the two cohorts. The prognostic performance of imaging biomarker S3 outperformed the whole tumor (C-index, 0.69 vs. 0.58; log-rank test, p < 0.001 vs. p = 0.552). Imaging biomarker S3 and AJCC stage were identified as independent predictors (p = 0.011 and 0.042, respectively) after adjusting for clinicopathological factors. The scoring system outperformed the traditional AJCC staging system (log-rank test, p < 0.0001 vs. p = 0.0002 in primary cohort and p = 0.0021 vs. p = 0.0277 in validation cohort, respectively).
Conclusions
Subregional radiomics analysis of PET/CT imaging has the potential to predict PFS in patients with NPC, which also provides complementary prognostic information for traditional predictors.





Similar content being viewed by others
Notes
Supervoxel refers to a small continuous region aggregated similar voxels with characteristics of PET, CT, PET local entropy, and CT local entropy.
References
Yu MC, Yuan JM (2002) Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 12:421–429
Cao SM, Simons MJ, Qian CN et al (2011) The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 30:114–119
Wei WI, Sham JS (2005) Nasopharyngeal carcinoma. Lancet 365:2041–2054
Yang ZH, Dai Q, Gu YJ, a. (2012) Cytokine and chemokine modification by Toll-like receptor polymorphisms is associated with nasopharyngeal carcinoma. Cancer Sci 103:653–658
Lu J, Chen XM, Huang HR et al (2018) Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck 40:1245–1253
Lin JC, Jan JS, Hsu CY et al (2003) Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 21:631–637
Lee AWM, Ma BBY, Ng WT, Chan ATC (2015) Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 33:3356–3364
Ng SH, Chan SC, Yen TC et al (2009) Staging of untreated nasopharyngeal carcinoma with PET/CT: comparison with conventional imaging work-up. Eur J Nucl Med Mol Imaging 36:12–22
Yen RF, Hong RL, Tzen KY et al (2005) Whole-body 18F-FDG PET in recurrent or metastatic nasopharyngeal carcinoma. J Nucl Med 46:770–774
Lin J, Xie G, Liao G et al (2017) Prognostic value of 18F-FDG-PET/CT in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Oncotarget 8:33884–33896
Ki CM, Jeong HS, Park Sang G et al (2009) Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 15:5861–5868
Paidpally V, Chirindel A, Chung CH et al (2014) FDG volumetric parameters and survival outcomes after definitive chemoradiotherapy in patients with recurrent head and neck squamous cell carcinoma. AJR Am J Roentgenol 203:W139–W145
Lambin P, Rios-Velazquez E, Leijenaar R et al (2007) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Chan SC, Chang KP, Fang YD et al (2016) Tumor heterogeneity measured on F-18 fluorodeoxyglucose positron emission tomography/computed tomography combined with plasma Epstein-Barr Virus load predicts prognosis in patients with primary nasopharyngeal carcinoma. Laryngoscope 127:E22–E28
Zhang B, Tian J, Dong D et al (2017) Radiomics features of multiparametric MRI as novel prognostic factors in advanced nasopharyngeal carcinoma. Clin Cancer Res 23:4259–4269
Ouyang FS, Guo BL, Zhang B et al (2017) Exploration and validation of radiomics signature as an independent prognostic biomarker in stage III-IVb nasopharyngeal carcinoma. Oncotarget 8:74869–74879
Lu L, Lv W, Jiang J et al (2016) Robustness of radiomic features in [11C]choline and [18F]FDG PET/CT imaging of nasopharyngeal carcinoma: impact of segmentation and discretization. Mol Imaging Biol 18:935–945
Lv W, Yuan Q, Wang Q et al (2018) Robustness versus disease differentiation when varying parameter settings in radiomics features: application to nasopharyngeal PET/CT. Eur Radiol 28:1–10
Lv W, Yuan Q, Wang Q et al (2019) Radiomics analysis of PET and CT components of PET/CT imaging integrated with clinical parameters: application to prognosis for nasopharyngeal carcinoma. Mol Imaging Biol. 21:954-964
Du D, Feng H, Lv W et al (2019) Machine learning methods for optimal radiomics-based differentiation between recurrence and inflammation: application to nasopharyngeal carcinoma post-therapy PET/CT images. Mol Imaging Biol. https://doi.org/10.1007/s11307-019-01411-9
Swanton C (2012) Intratumor heterogeneity: evolution through space and time. Cancer Res 72:4875–4882
Gatenby RA, Olya G, Gillies RJ (2013) Quantitative imaging in cancer evolution and ecology. Radiology 269:8–15
O'Connor JPB, Rose CJ, Waterton JC et al (2015) Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res 21:249–257
Wu J, Gensheimer MF, Dong X et al (2016) Robust intratumor partitioning to identify high-risk subregions in lung cancer: a pilot study. Int J Radiat Oncol Biol Phys 95:1504–1512
Even AJG, Reymen B, La Fontaine MD et al (2017) Clustering of multi-parametric functional imaging to identify high-risk subvolumes in non-small cell lung cancer. Radiother Oncol 125:379–384
Chaudhury B, Zhou M, Goldgof DB et al (2015) Heterogeneity in intratumoral regions with rapid gadolinium washout correlates with estrogen receptor status and nodal metastasis. J Magn Reson Imaging 42:1421–1430
Fan M, Cheng H, Zhang P et al (2017) DCE-MRI texture analysis with tumor subregion partitioning for predicting Ki-67 status of estrogen receptor-positive breast cancers. J Magn Reson Imaging 48:237–247
Xia W, Chen Y, Zhang R et al (2018) Radiogenomics of hepatocellular carcinoma: multiregion analysis-based identification of prognostic imaging biomarkers by integrating gene data a preliminary study. Phys Med Biol 63:170–179
Xie C, Yang P, Zhang X et al (2019) Sub-region based radiomics analysis for survival prediction in oesophageal tumours treated by definitive concurrent chemoradiotherapy. EBioMedicine 44:289–297
Ronald B, Roberto DB, Oyen WJG et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42:328–354
Kapp AV, Tibshirani R (2007) Are clusters found in one dataset present in another dataset? Biostatistics 8:9–31
Caliński T, Harabasz J (1974) A dendrite method for cluster analysis. Commun Stat 3:1–27
Zwanenburg A, Leger S, Vallières M, Löck S (2018) Image biomarker standardisation initiative. arXiv preprint arXiv:1612.07003v7
Wang X, Fritz A, Bent F (1994) Texture features from gray level gap length matrix. MVA'94 IAPR Workshop on Machine Vision Applications. 13-1 5. 1994. Kawasaki, Japan
Horng MH, Sun YN, Lin XZ (2002) Texture feature coding method for classification of liver sonography. Comput Med Imaging Graph 26:33–42
Sun C, Wee WG (1983) Neighboring gray level dependence matrix for texture classification. Computer Vision, Graphics & Image Processing 23:341–352
Rahmim A, Schmidtlein CR, Jackson A et al (2016) A novel metric for quantification of homogeneous and heterogeneous tumors in PET for enhanced clinical outcome prediction. Phys Med Biol 61:227–242
Foley KG, Hills RK, Berthon B et al (2018) Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur Radiol 28:428–436
Lao J, Chen Y, Li ZC et al (2017) A deep learning-based radiomics model for prediction of survival in glioblastoma multiforme. Sci Rep 7:10353
Bogowicz M, Leijenaar RTH, Tanadini-lang S et al (2017) Post-radiochemotherapy PET radiomics in head and neck cancer: the influence of radiomics implementation on the reproducibility of local control tumor models. Radiother Oncol 125:385–391
Wu J, Gong G, Cui Y, Li R (2016) Intratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapy. J Magn Reson Imaging 44:1107–1115
Eskey CJ, Koretsky AP, Domach MM, Jain RK (1992) 2H-Nuclear magnetic resonance imaging of tumor blood flow: spatial and temporal heterogeneity in a tissue-isolated mammary adenocarcinoma. Cancer Res 52:6010–6019
Choi YP, Shim HS, Gao MQ, Kang S, Cho NH (2011) Molecular portraits of intratumoral heterogeneity in human ovarian cancer. Cancer Lett 307:62–71
Gatenby RA, Silva AS, Gillies RJ, Frieden BR (2009) Adaptive therapy. Cancer Res 69:4894–4903
van Elmpt W, Zegers CML, Reymen B et al (2016) Multiparametric imaging of patient and tumour heterogeneity in non-small-cell lung cancer: quantification of tumour hypoxia, metabolism and perfusion. Eur J Nucl Med Mol Imaging 43:240–248
Mattonen SA, Davidzon GA, Bakr S et al (2019) [18F]FDG positron emission tomography (PET) Tumor and penumbra imaging features predict recurrence in non–small cell lung cancer. Tomography 5:145–153
Wu J, Cao G, Sun X, Lee J et al (2018) Intratumoral spatial heterogeneity at perfusion MR imaging predicts recurrence-free survival in locally advanced breast cancer treated with neoadjuvant chemotherapy. Radiology 288:26–35
Berry LR, Kai HB, Mary Ann G, Jed R et al (2010) Quantification of viable tumor microvascular characteristics by multispectral analysis. Magn Reson Med 60:64–72
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252
Pietrasab K (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316:1324–1331
Shimada S, Ohtsubo S, Ogasawara K, Kusano M (2015) Macro- and microscopic findings of ICG fluorescence in liver tumors. World J Surg Oncol 13:198
Funding
This work was supported by the National Natural Science Foundation of China under grants 81871437 and U1708261; the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (Lijun Lu, 2018); the Guangdong Basic and Applied Basic Research Foundation under grant 2019A1515011104; and the China Scholarship Council under grant 201808440464.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This retrospective study was approved by Institutional Review Boardand inform consent was waived.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 1322 kb)
Rights and permissions
About this article
Cite this article
Xu, H., Lv, W., Feng, H. et al. Subregional Radiomics Analysis of PET/CT Imaging with Intratumor Partitioning: Application to Prognosis for Nasopharyngeal Carcinoma. Mol Imaging Biol 22, 1414–1426 (2020). https://doi.org/10.1007/s11307-019-01439-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01439-x