Abstract
Purpose
The aim of this study was to evaluate different non-invasive methods for generating (R)-1-((3-([11C]methyl)pyridin-4-yl)methyl)-4-(3,4,5-trifluorophenyl)pyrrolidin-2-one) ([11C]UCB-J) parametric maps using white matter (centrum semi-ovale–SO) as reference tissue.
Procedures
Ten healthy volunteers (8 M/2F; age 27.6 ± 10.0 years) underwent a 90-min dynamic [11C]UCB-J positron emission tomography (PET) scan with full arterial blood sampling and metabolite analysis before and after administration of a novel chemical entity with high affinity for presynaptic synaptic vesicle glycoprotein 2A (SV2A). A simplified reference tissue model (SRTM2), multilinear reference tissue model (MRTM2), and reference Logan graphical analysis (rLGA) were used to generate binding potential maps using SO as reference tissue (BPSO). Shorter dynamic acquisitions down to 50 min were also considered. In addition, standard uptake value ratios (SUVR) relative to SO were evaluated for three post-injection intervals (SUVRSO,40-70min, SUVRSO,50-80min, and SUVRSO,60-90min respectively). Regional parametric BPSO + 1 and SUVRSO were compared with regional distribution volume ratios of a 1-tissue compartment model (1TCM DVRSO) using Spearman correlation and Bland-Altman analysis.
Results
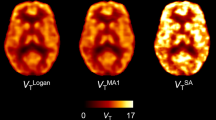
For all methods, highly significant correlations were found between regional, parametric BPSO + 1 (r = [0.63;0.96]) or SUVRSO (r = [0.90;0.91]) estimates and regional 1TCM DVRSO. For a 90-min dynamic scan, parametric SRTM2 and MRTM2 values presented similar small bias and variability (− 3.0 ± 2.9 % for baseline SRTM2) and outperformed rLGA (− 10.0 ± 5.3 % for baseline rLGA). Reducing the dynamic acquisition to 60 min had limited impact on the bias and variability of parametric SRTM2 BPSO estimates (− 1.0 ± 9.9 % for baseline SRTM2) while a higher variability (− 1.83 ± 10.8 %) for baseline MRTM2 was observed for shorter acquisition times. Both SUVRSO,60-90min and SUVRSO,50-80min showed similar small bias and variability (− 2.8 ± 4.6 % bias for baseline SUVRSO,60-90min).
Conclusion
SRTM2 is the preferred method for a voxelwise analysis of dynamic [11C]UCB-J PET using SO as reference tissue, while reducing the dynamic acquisition to 60 min has limited impact on [11C]UCB-J BPSO parametric maps. For a static PET protocol, both SUVRSO,60-90min and SUVRSO,50-80min images are an excellent proxy for [11C]UCB-J BPSO parametric maps.




Similar content being viewed by others
References
Crevecoeur J, Kaminski RM, Rogister B et al (2014) Expression pattern of synaptic vesicle protein 2 (SV2) isoforms in patients with temporal lobe epilepsy and hippocampal sclerosis. Neuropathol Appl Neurobiol 40:191–204
Feng G, Xiao F, Lu Y, Huang Z, Yuan J, Xiao Z, Xi Z, Wang X (2009) Down-regulation synaptic vesicle protein 2A in the anterior temporal neocortex of patients with intractable epilepsy. J Mol Neurosci 39:354–359
Proper EA, Oestreicher AB, Jansen GH, Veelen CWM, van Rijen PC, Gispen WH, de Graan PNE (2000) Immunohistochemical characterization of mossy fibre sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 123:19–30
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27:457–464
Hamos JE, DeGennaro LJ, Drachman DA (1989) Synaptic loss in Alzheimer’s disease and other dementias. Neurology 39:355–361
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson BS, Champagne F, Dwork AJ, Goldman J, Sulzer D (2014) Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83:1131–1143
Kang H, Voleti B, Hajszan T et al (2012) Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18:1413–1417
Glantz LA, Lewis DA (2000) Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. APN 57:65–73
Sekar A, Bialas AR, de Rivera H et al (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530:177–183
Bajjalieh SM, Frantz GD, Weimann JM, McConnell S, Scheller RH (1994) Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci 14:5223–5235
Finnema SJ, Nabulsi NB, Eid T et al (2016) Imaging synaptic density in the living human brain. Sci Transl Med 8:348–396
Nabulsi NB, Mercier J, Holden D, Carre S, Najafzadeh S, Vandergeten MC, Lin SF, Deo A, Price N, Wood M, Lara-Jaime T, Montel F, Laruelle M, Carson RE, Hannestad J, Huang Y (2016) Synthesis and preclinical evaluation of 11C-UCB-J as a PET tracer for imaging the synaptic vesicle glycoprotein 2A in the brain. J Nucl Med 57:777–784
Koole M, van Aalst J, Devrome M et al (2018) Quantifying SV2A density and drug occupancy in the human brain using [11C]UCB-J PET imaging and subcortical white matter as reference tissue. Eur J Nucl Med 1619–7089
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS (2003) Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 19:224–247
Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN (2010) Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab 30:46–50
Gunn R, Lammertsma A, Hume S et al (1997) Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 6(4):279–287
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE (2003) Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16:834–840
Wu Y, Carson RE (2002) Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22:1440–1452
Lammertsma (2017) Forward to the past: the case for quantitative PET imaging. J Nucl Med 58:1019–1024
Meyer PT, Hellwig S, Amtage F, Rottenburger C, Sahm U, Reuland P, Weber WA, Hull M (2011) Dual-biomarker imaging of regional cerebral amyloid load and neuronal activity in dementia with PET and 11C-labeled Pittsburgh compound B. J Nucl Med 52:393–400
Appel L, Jonasson M, Danfors T, Nyholm D, Askmark H, Lubberink M, Sorensen J (2015) Use of 11C-PE2I PET in differential diagnosis of parkinson disorders. J Nucl Med 56:234–242
Jonasson M, Appel L, Dansfors T et al (2017) Development of a clinically feasible [11C]PE2I PET method for differential diagnosis of parkinsonism using reduced scan duration and automated reference region extraction. Am J Nucl Med Mol Imaging 7:263–274
Peretti DE, Vallez Garcia D, Reesink FE et al (2019) Relative cerebral flow from dynamic PIB scans as an alternative for FDG scans in Alzheimer’s disease PET studies. PLoS One 14:e0211000
Slifstein M, Laruelle M (2000) Effects of statistical noise on graphic analysis of PET neuroreceptor studies. J Nucl Med 41:2083–2088
Finnema SJ, Nabulsi NB, Mercier J et al (2017) Kinetic evaluation and test-retest reproducibility of [11C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab 38:2041–2052
Van Laere K, Ahmad RU, Hudyana H et al (2013) Quantification of 18F-JNJ-42259152, a novel phosphodiesterase 10A PET tracer: kinetic modeling and test-retest study in human brain. J Nucl Med 54:1285–1293
Ottoy J, Verhaeghe J, Niemantsverdriet E, Engelborghs S, Stroobants S, Staelens S (2017) A simulation study on the impact of the blood flow-dependent component in [18F]AV45 SUVR in Alzheimer’s disease. PLoS One 12:e0189155
Cselényi Z, Farde L (2015) Quantification of blood flow-dependent component in estimates of beta-amyloid load obtained using quasi-steady-state standardized uptake value ratio. J Cereb Blood Flow Metab 35:1485–1493
van Berckel BN, Ossenkoppele R, Tolboom N et al (2013) Longitudinal amyloid imaging using 11C-PiB: methodologic considerations. J Nucl Med 54:1570–1576
Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, Perry R, O’Brien J (1999) White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry 67:66–67
Acknowledgments
The authors thank the participants in addition to the investigators and their teams who contributed to this study. We also thank Kwinten Porters and Jef Van Loock (department Nuclear Medicine, UZ Leuven) for their technical assistance, and the UZ Leuven radiopharmacy team for the tracer productions. We also acknowledge Barbara Pelgrims, PhD, (UCB Pharma, Brussels, BE) for publication coordination.
Funding
Part of this study was sponsored by a UCB Pharma research grant of UCB-J to KU Leuven (principal investigator Koen Van Laere).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ralph Paul Maguire, Brigitte Lacroix, Joel Mercier, and David Sciberras are employees of UCB Pharma. Nathalie Mertens, Kim Serdons, Koen Van Laere, and Michel Koole have no conflicts of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All subjects signed informed consent before entering the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 98 kb)
Rights and permissions
About this article
Cite this article
Mertens, N., Maguire, R.P., Serdons, K. et al. Validation of Parametric Methods for [11C]UCB-J PET Imaging Using Subcortical White Matter as Reference Tissue. Mol Imaging Biol 22, 444–452 (2020). https://doi.org/10.1007/s11307-019-01387-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01387-6