Abstract
Purpose
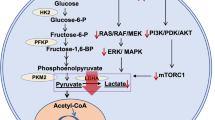
Pyruvate kinase M2 (PKM2) catalyzes the final step in glycolysis, the key process of tumor metabolism. PKM2 is found in high levels in glioblastoma (GBM) cells with marginal expression within healthy brain tissue, rendering it a key biomarker of GBM metabolic re-programming. Our group has reported the development of a novel radiotracer, 1-((2-fluoro- 6-[18F]fluorophenyl)sulfonyl)-4-((4-methoxyphenyl)sulfonyl)piperazine ([18F]DASA- 23), to non-invasively detect PKM2 levels with positron emission tomography (PET).
Procedure
U87 human GBM cells were treated with the IC50 concentration of various agents used in the treatment of GBM, including alkylating agents (temozolomide, carmustine, lomustine, procarbazine), inhibitor of topoisomerase I (irinotecan), vascular endothelial and epidermal growth factor receptor inhibitors (cediranib and erlotinib, respectively) anti-metabolite (5-fluorouracil), microtubule inhibitor (vincristine), and metabolic agents (dichloroacetate and IDH1 inhibitor ivosidenib). Following drug exposure for three or 6 days (n = 6 replicates per condition), the radiotracer uptake of [18F]DASA-23 and 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) was assessed. Changes in PKM2 protein levels were determined via Western blot and correlated to radiotracer uptake.
Results
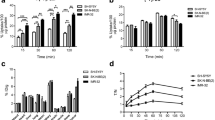
Significant interactions were found between the treatment agent (n = 12 conditions total comprised 11 drugs and vehicle) and the duration of treatment (3- or 6-day exposure to each drug) on the cellular uptake of [18F]DASA-23 (p = 0.0001). The greatest change in the cellular uptake of [18F]DASA-23 was found after exposure to alkylating agents (p < 0. 0001) followed by irinotecan (p = 0. 0012), erlotinib (p = 0. 02), and 5-fluorouracil (p = 0. 005). Correlation of PKM2 protein levels and [18F]DASA-23 cellular uptake revealed a moderate correlation (r = 0.44, p = 0.15).
Conclusions
These proof of principle studies emphasize the superiority of [18F]DASA-23 to [18F]FDG in detecting the glycolytic response of GBM to multiple classes of anti-neoplastic drugs in cell culture. A clinical trial evaluating the diagnostic utility of [18F]DASA-23 PET in GBM patients (NCT03539731) is ongoing.





Similar content being viewed by others
References
Adamson C, Kanu OO, Mehta AI, di C, Lin N, Mattox AK, Bigner DD (2009) Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 18:1061–1083
Roy S, Lahiri D, Maji T, Biswas J (2015) Recurrent glioblastoma: where we stand. South Asian J Cancer 4:163–173
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-Oncology 19:v1–v88
Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran DD, Brem S, Hottinger AF, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z (2017) Effect of tumor-treating fields plus maintenance Temozolomide vs maintenance Temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. J Am Med Assoc 318:2306–2316
Weller M, Cloughesy T, Perry JR, Wick W (2013) Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro-Oncology 15:4–27
Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW (2014) Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Investig New Drugs 32:452–464
Mellinghoff IK, Penas-Prado M, Peters KB, Cloughesy TF, Burris HA, Maher EA, Janku F, Cote GM, de la Fuente MI, Clarke J, Steelman L, le K, Zhang Y, Sonderfan A, Hummel D, Schoenfeld S, Yen K, Pandya SS, Wen PY (2018) Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH-mutant solid tumors, including glioma. J Clin Oncol 36:2002
Huang RY, Neagu MR, Reardon DA, Wen PY (2015) Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and Immuno/targeted therapy – detecting illusive disease, defining response. Front Neurol 6:33. https://doi.org/10.3389/fneur.2015.00033
Wen PY, Cloughesy TF, Ellingson BM, Reardon DA, Fine HA, Abrey L, Ballman K, Bendszuz M, Buckner J, Chang SM, Prados MD, Pope WB, Gregory Sorensen A, van den Bent M, Yung WKA (2014) Report of the jumpstarting brain tumor drug development coalition and FDA clinical trials neuroimaging endpoint workshop (January 30, 2014, Bethesda MD). Neuro-Oncology 16(Suppl 7):vii36–vii47
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, DeGroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF (2013) Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep 13:347
Ellingson BM, Wen PY, Cloughesy TF (2017) Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 14:307–320
Pope WB, Young JR, Ellingson BM (2011) Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 11:336–344
Hygino da Cruz LC, Rodriguez I, Domingues RC et al (2011) Pseudoprogression and Pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiol 32:1978–1985
Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2:683–693
Phelps ME (2000) Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci 97:9226–9233
Kelloff GJ, Hoffman JM, Johnson B et al (2005) Progress and promise of FDG-PET imaging for Cancer patient management and oncologic drug development. Clin Cancer Res 11:2785–2808
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougère C, Pope W, Law I, Arbizu J, Chamberlain MC, Vogelbaum M, Ellingson BM, Tonn JC (2016) Response assessment in neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology 18:1199–1208
Beinat C, Alam IS, James ML, Srinivasan A, Gambhir SS (2017) Development of [18F]DASA-23 for imaging tumor glycolysis through noninvasive measurement of pyruvate kinase M2. Mol Imaging Biol 19:665–672
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452:230–233
Agnihotri S, Zadeh G (2016) Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncology 18:160–172
Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol 8:839–847
Sharma R, Aboagye E (2011) Development of radiotracers for oncology--the interface with pharmacology. Br J Pharmacol 163:1565–1585
Witney TH, James ML, Shen B, Chang E, Pohling C, Arksey N, Hoehne A, Shuhendler A, Park JH, Bodapati D, Weber J, Gowrishankar G, Rao J, Chin FT, Gambhir SS (2015) PET imaging of tumor glycolysis downstream of hexokinase through noninvasive measurement of pyruvate kinase M2. Sci Transl Med 7:310ra169–310ra169
Perazzoli G, Prados J, Ortiz R, Caba O, Cabeza L, Berdasco M, Gónzalez B, Melguizo C (2015) Temozolomide resistance in glioblastoma cell lines: implication of MGMT, MMR, P-glycoprotein and CD133 expression. PLoS One 10:e0140131
Cowley GS, Weir BA, Vazquez F, Tamayo P, Scott JA, Rusin S, East-Seletsky A, Ali LD, Gerath WFJ, Pantel SE, Lizotte PH, Jiang G, Hsiao J, Tsherniak A, Dwinell E, Aoyama S, Okamoto M, Harrington W, Gelfand E, Green TM, Tomko MJ, Gopal S, Wong TC, Li H, Howell S, Stransky N, Liefeld T, Jang D, Bistline J, Hill Meyers B, Armstrong SA, Anderson KC, Stegmaier K, Reich M, Pellman D, Boehm JS, Mesirov JP, Golub TR, Root DE, Hahn WC (2014) Parallel genome-scale loss of function screens in 216 cancer cell lines for the identification of context-specific genetic dependencies. Scientific Data 1:140035
Gui DY, Lewis CA, Vander Heiden MG (2013) Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci Signal 6:pe7
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334:1278–1283
Park I, Mukherjee J, Ito M, Chaumeil MM, Jalbert LE, Gaensler K, Ronen SM, Nelson SJ, Pieper RO (2014) Changes in pyruvate metabolism detected by magnetic resonance imaging are linked to DNA damage and serve as a sensor of temozolomide response in glioblastoma cells. Cancer Res 74:7115–7124
Radoul M, Chaumeil MM, Eriksson P, Wang AS, Phillips JJ, Ronen SM (2016) MR studies of glioblastoma models treated with dual PI3K/mTOR inhibitor and Temozolomide:metabolic changes are associated with enhanced survival. Mol Cancer Ther 15:1113–1122
Rozental JM, Cohen JD, Mehta MP, Levine RL, Hanson JM, Nickles RJ (1993) Acute changes in glucose uptake after treatment: the effects of carmustine (BCNU) on human glioblastoma multiforme. J Neuro-Oncol 15:57–66
Guemes M, Rahman SA, Hussain K (2016) What is a normal blood glucose? Arch Dis Child 101:569–574
Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36:587–597
Daly ME, Vale C, Walker M, Littlefield A, Alberti KG, Mathers JC (1998) Acute effects on insulin sensitivity and diurnal metabolic profiles of a high-sucrose compared with a high-starch diet. Am J Clin Nutr 67:1186–1196
Kim MM, Parolia A, Dunphy MP, Venneti S (2016) Non-invasive metabolic imaging of brain tumours in the era of precision medicine. Nat Rev Clin Oncol 13:725–739
Lewis DY, Soloviev D, Brindle KM (2015) Imaging tumor metabolism using positron emission tomography. Cancer J 21:129–136
Pruim J, Willemsen AT, Molenaar WM, van Waarde A, Paans AM, Heesters MA, Go KG, Visser GM, Franssen EJ, Vaalburg W (1995) Brain tumors: L-[1-C-11]tyrosine PET for visualization and quantification of protein synthesis rate. Radiology 197:221–226
Bustany P, Chatel M, Derlon JM, Darcel F, Sgouropoulos P, Soussaline F, Syrota A (1986) Brain tumor protein synthesis and histological grades: a study by positron emission tomography (PET) with C11-L-methionine. J Neuro-Oncol 3:397–404
Juhász C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S (2014) Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging 13:7290.2014.00015. https://doi.org/10.2310/7290.2014.00015
Galldiks N, Rapp M, Stoffels G et al (2013) Earlier diagnosis of progressive disease during bevacizumab treatment using O-(2-18F-fluorethyl)-L-tyrosine positron emission tomography in comparison with magnetic resonance imaging. Mole Imaging 12:273–276
Galldiks N, Langen KJ, Holy R, Pinkawa M, Stoffels G, Nolte KW, Kaiser HJ, Filss CP, Fink GR, Coenen HH, Eble MJ, Piroth MD (2012) Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med 53:1048–1057
Patel CB, Fazzari E, Chakhoyan A, Yao J, Raymond C, Nguyen H, Manoukian J, Nguyen N, Pope W, Cloughesy TF, Nghiemphu PL, Czernin J, Lai A, Ellingson BM (2018) 18F-FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naive gliomas: a cross-sectional study. J Neuro-Oncol 139:399–409
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit Cancer cells? Trends Biochem Sci 41:211–218
Acknowledgments
We thank the Radiochemistry Facility at Stanford University for the 18F production, in particular Drs. Bin Shen, Jun Hyung Park and Jessa B. Castillo, and Mr. George Montoya for the [18F]FDG production.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors received funding from the following sources: Ben and Catherine Ivy Foundation (Gambhir), American Brain Tumor Association Basic Research Fellowship supported by the Ryan J. Hanrahan Memorial (Patel), Stanford Cancer Institute Fellowship for Cancer Research (Patel), Stanford-Asia Medical Fund C.J. Huang Medical Fellowship (Xie), Stanford School of Medicine Translational Research and Applied Medicine Fellowship (Beinat). The authors report no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1080 kb)
Rights and permissions
About this article
Cite this article
Beinat, C., Patel, C.B., Xie, Y. et al. Evaluation of Glycolytic Response to Multiple Classes of Anti-glioblastoma Drugs by Noninvasive Measurement of Pyruvate Kinase M2 Using [18F]DASA-23. Mol Imaging Biol 22, 124–133 (2020). https://doi.org/10.1007/s11307-019-01353-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01353-2