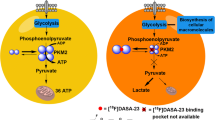
Abstract
Purpose
This study aims to explore whether 4-(2S,4R)-[18F]fluoroglutamine (4-[18F]FGln) positron emission tomography (PET) imaging is helpful in identifying and monitoring MYCN-amplified neuroblastoma by enhanced glutamine metabolism.
Procedures
Cell uptake studies and dynamic small-animal PET studies of 4-[18F]FGln and 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) were conducted in human MYCN-amplified (IMR-32 and SK-N-BE (2) cells) and non-MYCN-amplified (SH-SY5Y cell) neuroblastoma cells and animal models. Subsequently, short hairpin RNA (shRNA) knockdown of alanine-serine-cysteine transporter 2 (ASCT2/SLC1A5) in IMR-32 cells and xenografts were investigated in vitro and in vivo. Western blot (WB), real-time polymerase chain reaction (RT-PCR), and immunofluorescence (IF) assays were used to measure the prevalence of ASCT2, Ki-67, and c-Caspase 3, respectively.
Results
IMR-32 and SK-N-BE (2) cells showed high glutamine uptake in vitro (31.6 ± 1.7 and 21.6 ± 6.6 %ID/100 μg). In the in vivo study, 4-[18F]FGln was localized in IMR-32, SK-N-BE (2), and SH-SY5Y tumors with a high uptake (6.6 ± 0.3, 5.6 ± 0.2, and 3.7 ± 0.1 %ID/g). The maximum uptake (tumor-to-muscle, T/M) of the IMR-32 and SK-N-BE (2) tumors (3.71 and 2.63) was significantly higher than that of SH-SY5Y (1.54) tumors (P < 0.001, P < 0.001). The maximum uptake of 4-[18F]FGln in IMR-32 and SK-N-BE (2) tumors was 2.3-fold and 2.1-fold higher than that of [18F]FDG, respectively. Furthermore, in the in vitro and in vivo studies, the maximum uptake of 4-[18F]FGln in shASCT2-IMR-32 cells and tumors was 2.1-fold and 2.5-fold lower than that of the shControl-IMR-32. No significant difference in [18F]FDG uptake was found between shASCT2-IMR-32 and shControl-IMR-32 cells and tumors.
Conclusion
4-[18F]FGln PET can provide a valuable clinical tool in the assessment of metabolic glutamine uptake in MYCN-amplified neuroblastoma. ASCT2-targeted therapy may provide a supplementary method in MYCN-amplified neuroblastoma treatment.





Similar content being viewed by others
References
Luksch R, Castellani MR, Collini P, de Bernardi B, Conte M, Gambini C, Gandola L, Garaventa A, Biasoni D, Podda M, Sementa AR, Gatta G, Tonini GP (2016) Neuroblastoma (peripheral neuroblastic tumours). Crit Rev Oncol Hematol 107:163–181
Huang M, Weiss WA (2013) Neuroblastoma and MYCN. Cold Spring Harb Perspect Med 3:a014415
Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 178:93–105
Broer A, Rahimi F, Broer S (2016) Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. J Biol Chem 291:13194–13205
Fuchs BC, Bode BP (2005) Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 15:254–266
Janpipatkul K, Suksen K, Borwornpinyo S, Jearawiriyapaisarn N, Hongeng S, Piyachaturawat P, Chairoungdua A (2014) Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell Signal 26:1668–1679
Wasa M, Wang HS, Okada A (2002) Characterization of L-glutamine transport by a human neuroblastoma cell line. Am J Physiol Cell Physiol 282:C1246–C1253
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136:521–534
Boroughs LK, DeBerardinis RJ (2015) Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17:351–359
Wise DR, DeBerardinis RJ, Mancuso A, et al. (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105: 18782–18787
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, de Marzo AM, van Eyk JE, Mendell JT, Dang CV (2009) c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458:762–765
Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, Hogarty MD, Simon MC (2012) ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 22:631–644
Ren P, Yue M, Xiao D, Xiu R, Gan L, Liu H, Qing G (2015) ATF4 and N-Myc coordinate glutamine metabolism in MYCN amplified neuroblastoma cells through ASCT2 activation. J Pathol 235:90–100
Lieberman BP, Ploessl K, Wang L, Qu W, Zha Z, Wise DR, Chodosh LA, Belka G, Thompson CB, Kung HF (2011) PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J Nucl Med 52:1947–1955
Qu W, Zha Z, Ploessl K, Lieberman BP, Zhu L, Wise DR, B. Thompson C, Kung HF (2011) Synthesis of optically pure 4-fluoro-glutamines as potential metabolic imaging agents for tumors. J Am Chem Soc 133:1122–1133
Hassanein M, Hight MR, Buck JR, Tantawy MN, Nickels ML, Hoeksema MD, Harris BK, Boyd K, Massion PP, Manning HC (2016) Preclinical evaluation of 4-[18F]fluoroglutamine PET to assess ASCT2 expression in lung Cancer. Mol Imaging Biol 18:18–23
Schulte ML, Hight MR, Ayers GD, Liu Q, Shyr Y, Washington MK, Manning HC (2017) Non-invasive glutamine PET reflects pharmacological inhibition of BRAFV600E in vivo. Mol Imaging Biol 19:421–428
Venneti S, Dunphy MP, Zhang H et al (2015) Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med 7:274ra217
Zhu L, Ploessl K, Zhou R, Mankoff D, Kung HF (2017) Metabolic imaging of glutamine in cancer. J Nucl Med 58:533–537
Choi YJ, Hwang HS, Kim HJ, Jeong YH, Cho A, Lee JH, Yun M, Lee JD, Kang WJ (2014) 18F-FDG PET as a single imaging modality in pediatric neuroblastoma: comparison with abdomen CT and bone scintigraphy. Ann Nucl Med 28:304–313
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35:427–433
Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T, Henske EP, Haigis MC, Cantley LC, Stephanopoulos G, Yu J, Blenis J (2013) The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 153:840–854
Marquez J, Alonso FJ, Mates JM et al (2017) Glutamine addiction in gliomas. Neurochem Res 42:1735–1746
Choi YK, Park KG (2018) Targeting glutamine metabolism for cancer treatment. Biomol Ther (Seoul) 26:19–28
Effenberger M, Bommert KS, Kunz V, Kruk J, Leich E, Rudelius M, Bargou R, Bommert K (2017) Glutaminase inhibition in multiple myeloma induces apoptosis via MYC degradation. Oncotarget 8:85858–85867
Zhou R, Pantel AR, Li S, Lieberman BP, Ploessl K, Choi H, Blankemeyer E, Lee H, Kung HF, Mach RH, Mankoff DA (2017) [18F](2S,4R)4-fluoroglutamine PET detects glutamine pool size changes in triple-negative breast cancer in response to glutaminase inhibition. Cancer Res 77:1476–1484
Dang CV (2012) MYC on the path to cancer. Cell 149:22–35
Liu YL, Lu MY, Chang HH, Lu CC, Lin DT, Jou ST, Yang YL, Lee YL, Huang SF, Jeng YM, Lee H, Miser JS, Lin KH, Liao YF, Hsu WM, Tzen KY (2016) Diagnostic FDG and FDOPA positron emission tomography scans distinguish the genomic type and treatment outcome of neuroblastoma. Oncotarget 7:18774–18786
Ni Y, Zhou Y, Zhou M, Zhang L (2015) Akt and cAMP response element binding protein mediate 17beta-estradiol regulation of glucose transporter 3 expression in human SH-SY5Y neuroblastoma cell line. Neurosci Lett 604:58–63
Ploessl K, Wang L, Lieberman BP, Qu W, Kung HF (2012) Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J Nucl Med 53:1616–1624
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362:2202–2211
Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Yamada M, Oyama T, Takeyoshi I (2014) ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer 110:2030–2039
Acknowledgements
All authors thank the Institutional Technology Service Center of Shanghai Institute of Materia Medica for the technical support.
Funding
This work was supported by the National Natural Science Foundation (91859106, 81771890), the “Personalized Medicines—Molecular Signature-based Drug Discovery and Development,” Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12020108), and One Hundred Talent Program of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
CL and RH supervised the process of the study and performed the manuscript writing. HS, ZH, and CW performed the xenograft mice study and PET imaging. JG and LS are responsible for the radiolabeling of 4-[18F]FGln and [18F]FDG. CL and SH participated in western blot, real-time PCR work and IF. LL, CL, and LS are responsible for the data analysis. HW as scientific director has coordinated and approved the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, C., Huang, S., Guo, J. et al. Metabolic Evaluation of MYCN-Amplified Neuroblastoma by 4-[18F]FGln PET Imaging. Mol Imaging Biol 21, 1117–1126 (2019). https://doi.org/10.1007/s11307-019-01330-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01330-9