Abstract
Purpose
This study aimed to investigate the potential of apparent diffusion coefficient (ADC) for monitoring adipose-derived mesenchymal stem cell (ADMSC) therapy of renal ischemic-reperfusion injury (IRI).
Procedures
After baseline magnetic resonance imaging (MRI), 36 Sprague-Dawley rats with bilateral renal IRI were divided equally as groups 1, 2, and 3 (non-treated rats) and groups 4, 5, and 6 (ADMSC-treated rats, with 2 million ADMSCs injected via the tail vein at 6 h after IRI). Groups 1 and 4, 2 and 5, and 3 and 6 were euthanized at days 1, 3, and 7, respectively, after renal MRI. The ratios of ADC at different time points to baseline values in the cortex, outer, and inner stripes of outer medulla (OSOM/ISOM), assessments of monocyte chemoattractant protein-1 (MCP-1), CD68+ cells, tubular cast formation, and degree of fibrosis in three zones over time were compared between the non-treated and ADMSC-treated rats.
Results
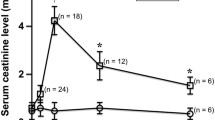
Among three zones, the differences in cortical ADC and immunohistochemical changes between the non-treated and ADMSC-treated IRI rats over time were less obvious. Compared with the non-treated rats, the ADMSC-treated rats exhibited significantly higher ADC ratios of OSOM and ISOM at days 1 and 3 corresponding to significantly less MCP-1 staining, CD68+ cells, and tubular casts. From day 3 to day 7, coupling with the decrement of MCP-1 and CD68+ cells in IRI kidneys, the effect of cell density on ADC declined. By day 7, the ADMSC-treated rats showed significantly higher ADC ratios of ISOM than the non-treated IRI rats, indicating better recovery, which could be related to significantly fewer tubular casts and marked amelioration of fibrosis.
Conclusions
We suggest ADC is a useful in vivo biomarker for monitoring ADMSC therapy of renal IRI.






Similar content being viewed by others
References
Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL (2006) Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 1:43–51
Lameire NH, Bagga A, Cruz D, de Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, van Biesen W, Vanholder R (2013) Acute kidney injury: an increasing global concern. Lancet 382:170–179
Hoste EA, Schurgers M (2008) Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 36:S146–S151
Bagul A, Frost JH, Drage M (2013) Stem cells and their role in renal ischaemia reperfusion injury. Am J Nephrol 37:16–29
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121:4210–4221
Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, Kuo HC, Pinkernell K (2010) Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant 25:3874–3884
Togel F, Weiss K, Yang Y et al (2007) Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292:F1626–F1635
Hu H, Zou C (2017) Mesenchymal stem cells in renal ischemia-reperfusion injury: biological and therapeutic perspectives. Curr Stem Cell Res Ther 12:183–187
Chen Y, Sun C, Lin Y et al (2011) Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med 9:51
Rowart P, Erpicum P, Detry O et al (2015) Mesenchymal stromal cell therapy in ischemia/reperfusion injury. J Immunol Res 2015:602597
Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K (2012) Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 156:785–795
Dewitte A, Coquin J, Meyssignac B, Joannès-Boyau O, Fleureau C, Roze H, Ripoche J, Janvier G, Combe C, Ouattara A (2012) Doppler resistive index to reflect regulation of renal vascular tone during sepsis and acute kidney injury. Crit Care 16:R165
Zhou HY, Chen TW, Zhang XM (2016) Functional magnetic resonance imaging in acute kidney injury: present status. Biomed Res Int 2016:2027370
Hueper K, Gutberlet M, Rong S, Hartung D, Mengel M, Lu X, Haller H, Wacker F, Meier M, Gueler F (2014) Acute kidney injury: arterial spin labeling to monitor renal perfusion impairment in mice-comparison with histopathologic results and renal function. Radiology 270:117–124
Zimmer F, Zöllner FG, Hoeger S, Klotz S, Tsagogiorgas C, Krämer BK, Schad LR (2013) Quantitative renal perfusion measurements in a rat model of acute kidney injury at 3T: testing inter- and intramethodical significance of ASL and DCE-MRI. PLoS One 8:e53849
Li LP, Thacker J, Lu J, Franklin T, Zhou Y, Papadopoulou MV, Solomon R, Prasad PV (2014) Efficacy of preventive interventions for iodinated contrast-induced acute kidney injury evaluated by intrarenal oxygenation as an early marker. Investig Radiol 49:647–652
Thoeny HC, De Keyzer F, Oyen RH et al (2005) Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 235:911–917
Taouli B, Thakur RK, Mannelli L, Babb JS, Kim S, Hecht EM, Lee VS, Israel GM (2009) Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology 251:398–407
Hueper K, Rong S, Gutberlet M, Hartung D, Mengel M, Lu X, Haller H, Wacker F, Meier M, Gueler F (2013) T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Investig Radiol 48:834–842
Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, Watanabe Y, Takenaka T, Katayama S, Tanaka J, Suzuki H (2011) Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 22:1429–1434
Ko SF, Yip HK, Zhen YY, Lee CC, Lee CC, Huang SJ, Huang CC, Ng SH, Lin JW (2017) Severe bilateral ischemic-reperfusion renal injury: hyperacute and acute changes in apparent diffusion coefficient, T1, and T2 mapping with immunohistochemical correlations. Sci Rep 7:1725
Donizetti-Oliveira C, Semedo P, Burgos-Silva M, Cenedeze MA, Malheiros DMAC, Reis MA, Pacheco-Silva A, Câmara NOS (2012) Adipose tissue-derived stem cell treatment prevents renal disease progression. Cell Transplant 21:1727–1741
Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G (2011) Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 26:1474–1483
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100:1249–1260
Schiffl H, Lang SM (2012) Update on biomarkers of acute kidney injury: moving closer to clinical impact? Mol Diagn Ther 16:199–207
Alge JL, Arthur JM (2015) Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 10:147–155
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:c179–c184
Mohsenin V (2017) Practical approach to detection and management of acute kidney injury in critically ill patient. J Intensive Care 5:57
Togao O, Doi S, Kuro-o M, Masaki T, Yorioka N, Takahashi M (2010) Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction. Radiology 255:772–780
Melgarejo E, Medina MA, Sánchez-Jiménez F et al (2009) Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol 41:998–1001
Winter JD, St Lawrence KS, Cheng HL (2011) Quantification of renal perfusion: comparison of arterial spin labeling and dynamic contrast-enhanced MRI. J Magn Reson Imaging 34:608–615
Brezis M, Rosen S (1995) Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332:647–655
Chin CL, Wehrli FW, Hwang SN et al (2002) Biexponential diffusion attenuation in the rat spinal cord: computer simulations based on anatomic images of axonal architecture. Magn Reson Med 47:455–460
Funding
This study was funded by the Ministry of Science and Technology, Taiwan (grant number MOST 103-2314-B-182A-066-MY3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Committee on Animal Care, Use and Research approved the experiment (IACUC number: 2013110102). All applicable institutional and/or national guidelines for the care and use of animals were followed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ko, SF., Yip, HK., Lee, CC. et al. Apparent Diffusion Coefficient is a Useful Biomarker for Monitoring Adipose-Derived Mesenchymal Stem Cell Therapy of Renal Ischemic-Reperfusion Injury. Mol Imaging Biol 20, 750–760 (2018). https://doi.org/10.1007/s11307-018-1184-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1184-0