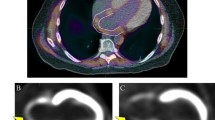
Abstract
Purpose
This study aims to analyze the left ventricular function parameters, scar load, and hypertrophy in a mouse model of pressure-overload left ventricular (LV) hypertrophy over the course of 8 weeks using 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) micro-positron emission tomography (microPET) imaging.
Procedures
LV hypertrophy was induced in C57BL/6 mice by transverse aortic constriction (TAC). Myocardial hypertrophy developed after 2–4 weeks. ECG-gated microPET scans with [18F]FDG were performed 4 and 8 weeks after surgery. The extent of fibrosis was measured by histopathologic analysis. LV function parameters and scar load were calculated using QGS®/QPS®. LV metabolic volume (LVMV) and percentage injected dose per gram were estimated by threshold-based analysis.
Results
The fibrotic tissue volume increased significantly from 4 to 8 weeks after TAC (1.67 vs. 3.91 mm3; P = 0.044). There was a significant increase of the EDV (4 weeks: 54 ± 15 μl, 8 weeks: 79 ± 32 μl, P < 0.01) and LVMV (4 weeks: 222 ± 24 μl, 8 weeks: 276 ± 52 μl, P < 0.01) as well as a significant decrease of the LVEF (4 weeks: 56 ± 17 %, 8 weeks: 44 ± 20 %, P < 0.01). The increase of LVMV had a high predictive value regarding the amount of ex vivo measured fibrotic tissue (R = 0.905, P < 0.001). The myocardial metabolic defects increased within 4 weeks (P = 0.055) but only moderately correlated with the fibrosis volume (R = 0.502, P = 0.021). The increase in end-diastolic volume showed a positive correlation with the fibrosis at 8 weeks (R = 0.763, P = 0.017).
Conclusions
[18F]FDG-PET is applicable for serial in vivo monitoring of the TAC mouse model. Myocardial hypertrophy, the dilation of the left ventricle, and the decrease in LVEF could be reliably quantified over time, as well as the developing localized scar. The increase in volume over time is predictive of a high fibrosis load.




Similar content being viewed by others
References
Levy D, Garrison RJ, Savage DD et al (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566
Burchfield JS, Xie M, Hill JA (2013) Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation 128:388–400
Marketou ME, Parthenakis F, Vardas PE (2016) Pathological left ventricular hypertrophy and stem cells: current evidence and new perspectives. Stem Cells Int 2016:5720758
Hein S, Arnon E, Kostin S et al (2003) Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 107:984–991
Katz AM (1990) Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med 322:100–110
Weidemann F, Herrmann S, Stork S et al (2009) Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 120:577–584
McLenachan JM, Dargie HJ (1990) Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens 3:735–740
Swynghedauw B, Chevalier B, Charlemagne D et al (1997) Cardiac hypertrophy, arrhythmogenicity and the new myocardial phenotype. II. The cellular adaptational process. Cardiovasc Res 35:6–12
Zhong M, Alonso CE, Taegtmeyer H, Kundu BK (2013) Quantitative PET imaging detects early metabolic remodeling in a mouse model of pressure-overload left ventricular hypertrophy in vivo. J Nucl Med 54:609–615
Coelho-Filho OR, Shah RV, Neilan TG et al (2014) Cardiac magnetic resonance assessment of interstitial myocardial fibrosis and cardiomyocyte hypertrophy in hypertensive mice treated with spironolactone. J Am Heart Assoc 3:e000790
Gross L, Paintmayer L, Lehner S et al (2016) FDG-PET reveals improved cardiac regeneration and attenuated adverse remodelling following Sitagliptin + G-CSF therapy after acute myocardial infarction. Eur Heart J Cardiovasc Imaging 17:136–145
Huber BC, Beetz NL, Laskowski A et al (2015) Attenuation of cardiac hypertrophy by G-CSF is associated with enhanced migration of bone marrow-derived cells. J Cell Mol Med 19:1033–1041
Lehner S, Todica A, Brunner S et al (2012) Temporal changes in phosphatidylserine expression and glucose metabolism after myocardial infarction: an in vivo imaging study in mice. Mol Imaging 11:461–470
Brunner S, Todica A, Boning G et al (2012) Left ventricular functional assessment in murine models of ischemic and dilated cardiomyopathy using [18 F]FDG-PET: comparison with cardiac MRI and monitoring erythropoietin therapy. Eur J Nucl Med Mol Iimaging Res 2:43
Boning G, Todica A, Vai A et al (2013) Erroneous cardiac ECG-gated PET list-mode trigger events can be retrospectively identified and replaced by an offline reprocessing approach: first results in rodents. Phys Med Biol 58:7937–7959
Lehner S, Todica A, Vanchev Y et al (2014) In vivo monitoring of parathyroid hormone treatment after myocardial infarction in mice with [68Ga]annexin A5 and [18F]fluorodeoxyglucose positron emission tomography. Mol Imaging 13
Todica A, Zacherl MJ, Wang H et al (2014) In-vivo monitoring of erythropoietin treatment after myocardial infarction in mice with [68Ga]Annexin A5 and [18F]FDG PET. J Nucl Cardiol 21:1191–1199
Croteau E, Benard F, Cadorette J et al (2003) Quantitative gated PET for the assessment of left ventricular function in small animals. J Nucl Med 44:1655–1661
Xia Y, Lee K, Li N et al (2009) Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 131:471–481
Kong EJ, Lee SH, Cho IH (2013) Myocardial fibrosis in hypertrophic cardiomyopathy demonstrated by integrated cardiac F-18 FDG PET/MR. Nucl Med Mol Imaging 47:196–200
Maron MS, Olivotto I, Maron BJ et al (2009) The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 54:866–875
Olivotto I, Cecchi F, Gistri R et al (2006) Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol 47:1043–1048
Petersen SE, Jerosch-Herold M, Hudsmith LE et al (2007) Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 115:2418–2425
Cecchi F, Olivotto I, Gistri R et al (2003) Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 349:1027–1035
Davis J, Molkentin JD (2014) Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70:9–18
Anderson CD, Heydarkhan-Hagvall S, Schenke-Layland K et al (2008) The role of cytoprotective cytokines in cardiac ischemia/reperfusion injury. J Surg Res 148:164–171
Chimenti I, Smith RR, Li TS et al (2010) Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res 106:971–980
Leri A, Kajstura J, Anversa P (2011) Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res 109:941–961
Theiss HD, Vallaster M, Rischpler C et al (2011) Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis. Stem Cell Res 7:244–255
Bishop SP, Altschuld RA (1970) Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Phys 218:153–159
Doenst T, Pytel G, Schrepper A et al (2010) Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res 86:461–470
Young ME, Yan J, Razeghi P et al (2007) Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Bio 1:251–262
Kundu BK, Zhong M, Sen S et al (2015) Remodeling of glucose metabolism precedes pressure overload-induced left ventricular hypertrophy: review of a hypothesis. Cardiology 130:211–220
Fueger BJ, Czernin J, Hildebrandt I et al (2006) Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 47:999–1006
Kreissl MC, Stout DB, Wong KP et al (2011) Influence of dietary state and insulin on myocardial, skeletal muscle and brain [F]-fluorodeoxyglucose kinetics in mice. Eur J Nucl Med Mol Imaging Res 1:8
Acknowledgements
A part of this work originated from the doctoral thesis of Nick L. Beetz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Todica, A., Beetz, N.L., Günther, L. et al. Monitoring of Cardiac Remodeling in a Mouse Model of Pressure-Overload Left Ventricular Hypertrophy with [18F]FDG MicroPET. Mol Imaging Biol 20, 268–274 (2018). https://doi.org/10.1007/s11307-017-1114-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1114-6