Abstract
Purpose
Plaque vulnerability is associated with inflammation and angiogenesis, processes that rely on vascular endothelial growth factor (VEGF) signaling via two receptors, VEGFR-1 and VEGFR-2. We have recently reported that enhanced uptake of scVEGF-PEG-DOTA/Tc-99m (scV/Tc) single photon emission computed tomography (SPECT) tracer that targets both VEGFR-1 and VEGFR-2, identifies accelerated atherosclerosis in diabetic relative to non-diabetic ApoE−/− mice. Since VEGFR-1 and VEGFR-2 may play different roles in atherosclerotic plaques, we reasoned that selective imaging of each receptor can provide more detailed information on plaque biology.
Procedures
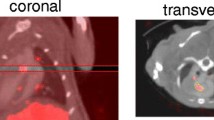
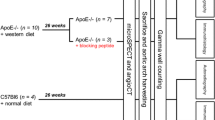
Recently described VEGFR-1 and VEGFR-2 selective mutants of scVEGF, named scVR1 and scVR2, were site-specifically derivatized with Tc-99m chelator DOTA via 3.4 kDa PEG linker, and their selectivity to the cognate receptors was confirmed in vitro. scVR1 and scVR2 conjugates were radiolabeled with Tc-99m to specific activity of 110 ± 11 MBq/nmol, yielding tracers named scVR1/Tc and scVR2/Tc. 34–40 week old diabetic and age-matched non-diabetic ApoE−/− mice were injected with tracers, 2–3 h later injected with x-ray computed tomography (CT) contrast agent and underwent hybrid SPECT/CT imaging. Tracer uptake, localized to proximal aorta and brachiocephalic vessels, was quantified as %ID from. Tracer uptake was also quantified as %ID/g from gamma counting of harvested plaques. Harvested atherosclerotic arterial tissue was used for immunofluorescent analyses of VEGFR-1 and VEGFR-2 and various lineage-specific markers.
Results
Focal, receptor-mediated uptake in proximal aorta and brachiocephalic vessels was detected for both scVR1/Tc and scVR2/Tc tracers. Uptake of scVR1/Tc and scVR2/Tc was efficiently inhibited only by “cold” proteins of the same receptor selectivity. Tracer uptake in this area, expressed as %ID, was higher in diabetic vs. non- diabetic mice for scVR1/Tc (p = 0.01) but not for scVR2/Tc. Immunofluorescent analysis revealed enhanced VEGFR-1 prevalence in and around plaque area in diabetic mice.
Conclusions
Selective VEGFR-1 and VEGFR-2 imaging of atherosclerotic lesions may be useful to explore plaque biology and identify vulnerability.





Similar content being viewed by others
References
Finn AV, Nakano M, Narula J et al (2010) Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 30:1282–1292
Kusters DH, Tegtmeier J, Schurgers LJ, Reutelingsperger CP (2012) Molecular imaging to identify the vulnerable plaque—from basic research to clinical practice. Mol Imaging Biol 14:523–533
Quillard T, Libby P (2012) Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res 111:231–244
Holm PW, Slart RH, Zeebregts CJ et al (2009) Atherosclerotic plaque development and instability: a dual role for VEGF. Ann Med 41:257–264
Liu H, Wang X, Tan KB et al (2011) Molecular imaging of vulnerable plaques in rabbits using contrast-enhanced ultrasound targeting to vascular endothelial growth factor receptor-2. J Clin Ultrasound 39:83–90
Golestani R, Zeebregts CJ, Terwisscha van Scheltinga AG et al (2013) Feasibility of vascular endothelial growth factor imaging in human atherosclerotic plaque using (89)Zr-bevacizumab positron emission tomography. Mol Imag 12:235–243
Lam MK, Al-Ansari S, Gooitzen M (2013) Single-chain VEGF/Cy5.5 targeting VEGF receptors to indicate atherosclerotic plaque instability. Mol Imaging Biol 15:250–261
Tekabe Y, Kollaros M, Zerihoun A et al (2014) Imaging VEGF receptor expression to identify accelerated atherosclerosis. EJNMMI Res 4:41–49
Backer MV, Levashova Z, Patel V et al (2007) Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med 13:504–509
Meyer J-P, Edwards KJ, Kozlowski P et al (2016) Selective imaging of VEGFR-1 and VEGFR-2 receptors using 89Zr-labeled single-chain VEGF mutants. J Nucl Med 57:1811–1816
Chistiakov DA, Bobryshev YV, Orekhov AN (2015) Changes in transcriptome of macrophages in atherosclerosis. J Cell Mol Med 19:1163–1173
Shashkin P, Dragulev B, Ley K (2005) Macrophage differentiation to foam cells. Curr Pharm Des 11:3061–3072
Fukuda D, Aikawa M (2013) Expanding role of delta-like 4 mediated notch signaling in cardiovascular and metabolic diseases. Circul J 77:2462–2468
Gori JL, Butler JM, Chan YY et al (2015) Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Inv 125:1243–1254
Rahimi N (2006) VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci 11:818–829
Shankman LS, Gomez D, Cherepanova OA et al (2015) KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 21(6):628–637
Michel JB, Martin-Ventura JL, Nicoletti A, Ho-Tin-Noe B (2014) Pathology of human plaque vulnerability mechanisms and consequences of intraplaque haemorrhages. Atheroscler 234(2):311–319
Virmani R, Kolodgie FD, Burke AP et al (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 5(10):2054–2061
Getz GS, Reardon CA (2012) Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol 32:1104–1115
Acknowledgments
This work was supported in part by NIH SBIR Phase I contract HHSN26801400041C to JMB and LLJ. We thank Dr. Lucrecia Marques-Rosado for help with immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Joseph Backer is a shareholder in SibTech, Inc.
Electronic supplementary material
ESM 1
(PDF 211 kb)
Rights and permissions
About this article
Cite this article
Tekabe, Y., Johnson, L.L., Rodriquez, K. et al. Selective Imaging of Vascular Endothelial Growth Factor Receptor-1 and Receptor-2 in Atherosclerotic Lesions in Diabetic and Non-diabetic ApoE−/− Mice. Mol Imaging Biol 20, 85–93 (2018). https://doi.org/10.1007/s11307-017-1045-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1045-2