Abstract
Purpose
Vulnerable plaques are key factors for ischemic diseases. Thus, their precise detection is necessary for the diagnosis of such diseases. Immunoglobulin G (IgG)-based imaging probes have been developed for imaging biomolecules related to plaque formation for the diagnosis of atherosclerosis. However, IgG accumulates nonspecifically in atherosclerotic regions, and its accumulation mechanisms have not yet been clarified in detail. Therefore, we explored IgG accumulation mechanisms in atherosclerotic lesions and examined images of radiolabeled IgG for the diagnosis of atherosclerosis.
Procedures
Mouse IgG without specificity to biomolecules was labeled with technetium-99m via 6-hydrazinonicotinate to yield [99mTc]IgG. ApoE−/− or C57BL/6J mice were injected intravenously with [99mTc]IgG, and their aortas were excised 24 h after injection. After radioactivity measurement, serial aortic sections were autoradiographically and histopathologically examined. RAW264.7 macrophages were polarized into M1 or M2 and then treated with [99mTc]IgG. The radioactivities in the cells were measured after 1 h of incubation. [99mTc]IgG uptake in M1 macrophages was also evaluated after the pretreatment with an anti-Fcγ receptor (FcγR) antibody. The expression levels of FcγRs in the cells were measured by western blot analysis.
Results
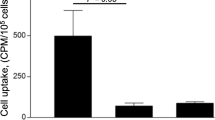
[99mTc]IgG accumulation levels in the aortas were significantly higher in apoE−/− mice than in C57BL/6J mice (5.1 ± 1.4 vs 2.8 ± 0.5 %ID/g, p < 0.05). Autoradiographic images showed that the accumulation areas highly correlated with the macrophage-infiltrated areas. M1 macrophages showed significantly higher levels of [99mTc]IgG than M2 or M0 (nonpolarized) macrophages [2.2 ± 0.3 (M1) vs 0.5 ± 0.1 (M2), 0.4 ± 0.1 (M0) %dose/mg protein, p < 0.01] and higher expression levels of FcγRI and FcγRII. [99mTc]IgG accumulation in M1 macrophages was suppressed by pretreatment with the anti-FcγR antibody [2.2 ± 0.3 (nonpretreatment) vs 1.2 ± 0.2 (pretreatment) %ID/mg protein, p < 0.01].
Conclusions
IgG accumulated in pro-inflammatory M1 macrophages via FcγRs in atherosclerotic lesions. Thus, the target biomolecule-independent imaging of active inflammation should be taken into account in the diagnosis of atherosclerosis using IgG-based probes.





Similar content being viewed by others
References
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92:657–671
Hanzawa H, Sakamoto T, Kaneko A et al (2015) Combined plasma and tissue proteomic study of atherogenic model mouse: approach to elucidate molecular determinants in atherosclerosis development. J Proteome Res 14:4257–4269
Wang X, Connolly TM (2010) Biomarkers of vulnerable atheromatous plaques: translational medicine perspectives. Adv Clin Chem 50:1–22
Sakamoto T, Hanzawa H, Manri N et al (2016) Discovery and evaluation of biomarkers for atherosclerosis. In: Kuge Y, Shiga T, Tamaki N (eds) Perspectives on nuclear medicine for molecular diagnosis and integrated therapy. Springer Japan, Sapporo, pp. 131–139
Tarkin JM, Joshi FR, Rudd JH (2014) PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 11:443–457
Bucerius J, Hyafil F, Verberne HJ et al (2016) Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging 43:780–792
Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105:1135–1143
Shimizu Y, Kuge Y (2016) Recent advances in the development of PET/SPECT probes for atherosclerosis imaging. Nucl Med Mol Imaging 50:284–291
Temma T, Saji H (2012) Radiolabelled probes for imaging of atherosclerotic plaques. Am J Nucl Med Mol Imaging 2:432–447
Nakamura I, Hasegawa K, Wada Y, Hirase T, Node K, Watanabe Y (2013) Detection of early stage atherosclerotic plaques using PET and CT fusion imaging targeting P-selectin in low density lipoprotein receptor-deficient mice. Biochem Biophys Res Commun 433:47–51
Temma T, Ogawa Y, Kuge Y et al (2010) Tissue factor detection for selectively discriminating unstable plaques in an atherosclerotic rabbit model. J Nucl Med 51:1979–1986
Kuge Y, Takai N, Ogawa Y et al (2010) Imaging with radiolabelled anti-membrane type 1 matrix metalloproteinase (MT1-MMP) antibody: potentials for characterizing atherosclerotic plaques. Eur J Nucl Med Mol Imaging 37:2093–2104
Larson SM (1985) Radiolabeled monoclonal anti-tumor antibodies in diagnosis and therapy. J Nucl Med 26:538–545
McCafferty J, Griffiths AD, Winter G, Chiswell DJ (1990) Phage antibodies: filamentous phage displaying antibody variable domains. 348:552–554
Demacker PN, Dormans TP, Koenders EB, Corstens FH (1993) Evaluation of indium-111-polyclonal immunoglobulin G to quantitate atherosclerosis. J Nucl Med 34:1316–1321
Fischman AJ, Rubin RH, Khaw BA et al (1989) Radionuclide imaging of experimental atherosclerosis with nonspecific polyclonal immunoglobulin G. J Nucl Med 30:1095–1100
Shimizu Y, Hanzawa H, Zhao Y et al (2016) Radioimmunodetection of atherosclerotic lesions focusing on the accumulation mechanism of immunoglobulin G. In: Kuge Y, Shiga T, Tamaki N (eds) Perspectives on nuclear medicine for molecular diagnosis and integrated therapy. Springer Japan, Sapporo, pp. 141–150
De Gersem R, Jamar F (2010) Nonspecific human immunoglobulin G for imaging infection and inflammation: what did we learn? The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So 54:617–628
Fischman AJ, Rubin RH, White JA et al (1990) Localization of Fc and Fab fragments of nonspecific polyclonal IgG at focal sites of inflammation. J Nucl Med 31:1199–1205
Abrams MJ, Juweid M, tenKate CI et al (1990) Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J Nucl Med 31:2022–2028
Ono M, Arano Y, Mukai T et al (2001) Plasma protein binding of (99 m)Tc-labeled hydrazino nicotinamide derivatized polypeptides and peptides. Nucl Med Biol 28:155–164
Larsen SK, Solomon HF, Caldwell G, Abrams MJ (1995) [99mTc]tricine: a useful precursor complex for the radiolabeling of hydrazinonicotinate protein conjugates. Bioconjug Chem 6:635–638
Vucic E, Dickson SD, Calcagno C et al (2011) Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. JACC Cardiovasc Imaging 4:1100–1109
Zhao Y, Kuge Y, Zhao S et al (2007) Comparison of 99mTc-annexin A5 with 18F-FDG for the detection of atherosclerosis in ApoE−/− mice. Eur J Nucl Med Mol Imaging 34:1747–1755
Zhang B, Wang J, Gao J et al (2009) Alternatively activated RAW264.7 macrophages enhance tumor lymphangiogenesis in mouse lung adenocarcinoma. J Cell Biochem 107:134–143
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Chinetti-Gbaguidi G, Baron M, Bouhlel MA et al (2011) Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res 108:985–995
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13:709–721
van de Winkel JG, Anderson CL (1991) Biology of human immunoglobulin G Fc receptors. J Leukoc Biol 49:511–524
Anderson CF, Mosser DM (2002) Cutting edge: biasing immune responses by directing antigen to macrophage Fc gamma receptors. J Immunol (Baltimore, Md : 1950) 168:3697–3701
Ravetch JV, Bolland S (2001) IgG Fc receptors. Annu Rev Immunol 19:275–290
Ogawa M, Ishino S, Mukai T et al (2004) 18F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 45:1245–1250
Satomi T, Ogawa M, Mori I et al (2013) Comparison of contrast agents for atherosclerosis imaging using cultured macrophages: FDG versus ultrasmall superparamagnetic iron oxide. J Nucl Med 54:999–1004
Ziegler WAWSI, Tho’dtmann R, Hanauske A-R, Schwaiger M (1999) Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med 40:1771–1777
Zhao Y, Zhao S, Kuge Y, Tamaki N (2013) Elevated 18F-FDG levels in blood and organs after angiotensin II receptor blocker administration: experiment in mice administered telmisartan. J Nucl Med 54:1384–1388
Cuesta AM, Sainz-Pastor N, Bonet J, Oliva B, Alvarez-Vallina L (2010) Multivalent antibodies: when design surpasses evolution. Trends Biotechnol 28:355–362
Broisat A, Hernot S, Toczek J et al (2012) Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ Res 110:927–937
Ogawa M, Magata Y, Kato T et al (2006) Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med 47:1845–1850
Zhao Y, Fukao K, Zhao S et al (2015) Irbesartan attenuates atherosclerosis in Watanabe heritable hyperlipidemic rabbits: noninvasive imaging of inflammation by 18F-fluorodeoxyglucose positron emission tomography. Mol Imaging 14. doi:10.2310/7290.2015.00004
Romer T, Leonhardt H, Rothbauer U (2011) Engineering antibodies and proteins for molecular in vivo imaging. Curr Opin Biotech 22:882–887
Acknowledgements
This study was supported by JSPS KAKENHI (Grant Numbers 26293268, 15K15440, 24890003, and 26860961) and the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
N.T. has grant support from Hitachi, Ltd. H.H. and T.S. are employees of Hitachi Ltd. The other authors declare that there is no conflict of interest associated with this manuscript.
Electronic supplementary material
ESM 1
(PDF 143 kb)
Rights and permissions
About this article
Cite this article
Shimizu, Y., Hanzawa, H., Zhao, Y. et al. Immunoglobulin G (IgG)-Based Imaging Probe Accumulates in M1 Macrophage-Infiltrated Atherosclerotic Plaques Independent of IgG Target Molecule Expression. Mol Imaging Biol 19, 531–539 (2017). https://doi.org/10.1007/s11307-016-1036-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-1036-8