Abstract
Purpose
In oncology, positron emission tomography imaging using dedicated tracers as biomarkers may assist in early evaluation of therapy efficacy. Using 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT), we investigated the early effects of chemotherapeutic treatment on cancer cell proliferation in a BRAF-mutated colorectal cancer xenograft model.
Procedures
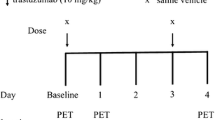
Colo205 subcutaneously inoculated animals underwent 90-min dynamic imaging before and 24 h after treatment with vehicle (control), cetuximab (resistant) or irinotecan (sensitive). Total distribution volume was quantified from dynamic data, and standardized uptake values as well as tumor-to-blood ratios were calculated from static images averaged over the last 20 min. In vivo imaging data was correlated with ex vivo proliferation and thymidine metabolism proteins.
Results
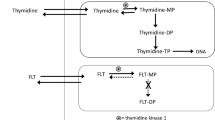
All imaging parameters showed a significant post-treatment decrease from [18F]FLT baseline uptake for the irinotecan group (p ≤ 0.001) as compared with the cetuximab and vehicle group and correlated strongly with each other (p ≤ 0.0001). In vivo data were in agreement with Ki67 staining, showing a significantly lower percentage of Ki67-positive cells in the irinotecan group as compared with other groups (p ≤ 0.0001). Tumor expression of thymidine kinase 1 phosphorylated on serine 13, thymidylate synthase, and thymidine phosphorylase remained unaffected, while thymidine kinase 1 expression was, surprisingly, significantly higher in irinotecan-treated animals (p ≤ 0.01). In contrast, tumor ATP levels were lowest in this group.
Conclusions
[18F]FLT positron emission tomography was found to be a suitable biomarker of early tumor response to anti-proliferative treatment, with static imaging not being inferior to full compartmental analysis in our xenograft model. The dynamics of thymidine kinase 1 protein expression and protein activity in low ATP environments merits further investigation.






Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386
Shields AF, Grierson JR, Dohmen BM et al (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 4:1334–1336
Bading JR, Shields AF (2008) Imaging of cell proliferation: status and prospects. J Nucl Med 49:64S–80S
Hu C-M, Chang Z-F (2007) Mitotic control of dTTP pool: a necessity or coincidence? J Biomed Sci 14:491–497
Munch-Petersen B, Cloos L, Jensen HK, Tyrsted G (1995) Human thymidine kinase 1. Regulation in normal and malignant cells. Adv Enzym Regul 35:69–89
Li C-L, Lu C-Y, Ke P-Y, Chang Z-F (2004) Perturbation of ATP-induced tetramerization of human cytosolic thymidine kinase by substitution of serine-13 with aspartic acid at the mitotic phosphorylation site. Biochem Biophys Res Commun 313:587–593
Soloviev D, Lewis D, Honess D, Aboagye E (2012) [(18)F]FLT: an imaging biomarker of tumour proliferation for assessment of tumour response to treatment. Eur J Cancer 48:416–424
Mudd SR, Holich KD, Voorbach MJ et al (2011) Pharmacodynamic evaluation of irinotecan therapy by FDG and FLT PET/CT imaging in a colorectal cancer xenograft model. Mol Imaging Biol 14:617–624
McKinley ET, Smith RA, Zhao P et al (2013) 3′-Deoxy-3′-18F-fluorothymidine pet predicts response to V600EBRAF-targeted therapy in preclinical models of colorectal cancer. J Nucl Med 54:424–430
Tomasi G, Turkheimer F, Aboagye E (2011) Importance of quantification for the analysis of PET data in oncology: review of current methods and trends for the future. Mol Imaging Biol 14:131–146
Gunn RN, Gunn SR, Cunningham VJ (2001) Positron emission tomography compartmental models. J Cereb Blood Flow Metab 21:635–652
Kim SJ, Lee JS, Im KC et al (2008) Kinetic modeling of 3“-deoxy-3-”18F-fluorothymidine for quantitative cell proliferation imaging in subcutaneous tumor models in mice. J Nucl Med 49:2057–2066
Jonker DJ, O’Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Di Nicolantonio F, Martini M, Molinari F et al (2008) Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 26:5705–5712
Pietrantonio F, Petrelli F, Coinu A et al (2015) Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer 51:587–594
Rothenberg ML (2001) Irinotecan (CPT-11): recent developments and future directions—colorectal cancer and beyond. Oncologist 6:66–80
Voeller DM, Grem JL, Pommier Y et al (2000) Identification and proposed mechanism of action of thymidine kinase inhibition associated with cellular exposure to camptothecin analogs. Cancer Chemother Pharmacol 45:409–416
Visser EP, Disselhorst JA, Brom M et al (2009) Spatial resolution and sensitivity of the Inveon small-animal PET scanner. J Nucl Med 50:139–147
Deleye S, Heylen M, Deiteren A et al (2014) Continuous flushing of the bladder in rodents reduces artifacts and improves quantification in molecular imaging. Mol Imaging 13:1–12
Brockenbrough JS, Morihara JK, Hawes SE et al (2009) Thymidine kinase 1 and thymidine phosphorylase expression in non-small-cell lung carcinoma in relation to angiogenesis and proliferation. J Histochem Cytochem 57:1087–1097
Lee W-C, Chang C-H, Ho C-L et al (2011) Early detection of tumor response by FLT/MicroPET imaging in a C26 murine colon carcinoma solid tumor animal model. J Biomed Biotechnol 2011:1–7
Troost EGC, Bussink J, Hoffmann AL et al (2010) 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med 51:866–874
Frings V, Yaqub M, Hoyng LL et al (2014) Assessment of simplified methods to measure 18F-FLT uptake changes in EGFR-mutated non-small cell lung cancer patients undergoing EGFR tyrosine kinase inhibitor treatment. J Nucl Med 55:1417–1423
Brockenbrough JS, Souquet T, Morihara JK et al (2011) Tumor 3“-deoxy-3-”18F-fluorothymidine (18F-FLT) uptake by PET correlates with thymidine kinase 1 expression: static and kinetic analysis of 18F-FLT PET studies in lung tumors. J Nucl Med 52:1181–1188
Vesselle H, Grierson J, Muzi M et al (2002) In vivo validation of 3′-deoxy-3′-[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 8:3315–3323
McKinley ET, Ayers GD, Smith RA et al (2013) Limits of [18F]-FLT PET as a biomarker of proliferation in oncology. PLoS ONE 8:e58938
Zhang CC, Yan Z, Li W et al (2012) [18F]FLT-PET imaging does not always “light up” proliferating tumor cells. Clin Cancer Res 18:1303–1312
Keen H, Pichler B, Kukuk D et al (2011) An evaluation of 2-deoxy-2-[18F]fluoro-D-glucose and 3′-deoxy-3′-[18F]-fluorothymidine uptake in human tumor xenograft models. Mol Imaging Biol 14:355–365
Lee SJ, Kim SY, Chung JH et al (2010) Induction of thymidine kinase 1 after 5-fluorouracil as a mechanism for 3“-deoxy-3-”[18F]fluorothymidine flare. Biochem Pharmacol 80:1528–1536
Kenny LM, Contractor KB, Stebbing J et al (2009) Altered tissue 3“-deoxy-3-”[18F]fluorothymidine pharmacokinetics in human breast cancer following capecitabine treatment detected by positron emission tomography. Clin Cancer Res 15:6649–6657
Barthel H, Cleij MC, Collingridge DR et al (2003) 3“-Deoxy-3-”[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res 63:3791–3798
Chang ZF, Huang DY, Chi LM (1998) Serine 13 is the site of mitotic phosphorylation of human thymidine kinase. J Biol Chem 273:12095–12100
Segura-Peña D, Lutz S, Monnerjahn C et al (2007) Binding of ATP to TK1-like enzymes is associated with a conformational change in the quaternary structure. J Mol Biol 369:129–141
Acknowledgments
The authors thank Philippe Joye and Caroline Berghmans of the Molecular Imaging Center Antwerp for their valuable technical assistance.
This work was funded by the University of Antwerp through a Bijzonder Onderzoeksfond (BOF27327). D.T. is funded by the Research Foundation Flanders (FWO) through a postdoctoral grant (1211313N). Si.S and C.V. are supported by the Innovative Medicines Initiative Joint Undertaking (www.imi.europa.eu) under grant agreement number 115151, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in-kind contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 566 kb)
Rights and permissions
About this article
Cite this article
Rapic, S., Vangestel, C., Verhaeghe, J. et al. Evaluation of [18F]Fluorothymidine as a Biomarker for Early Therapy Response in a Mouse Model of Colorectal Cancer. Mol Imaging Biol 19, 109–119 (2017). https://doi.org/10.1007/s11307-016-0974-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-0974-5