Abstract
Purpose
Prefrontal cortex (PFC) deep brain stimulation (DBS) has been proposed as a therapy for addiction and depression. This study investigates changes in rat cerebral glucose metabolism induced by different DBS frequencies using μPET.
Procedures
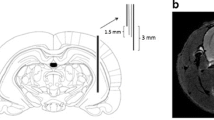
One hour DBS of the prelimbic area (PL) of the medial PFC (mPFC) (60 Hz, 130 Hz or sham) in rats (n = 9) was followed by 2-deoxy-2-[18 F] fluoro-d-glucose ([18 F]FDG) μPET.
Results
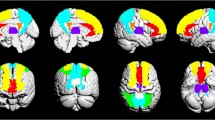
Sixty Hz DBS elicited significant hypermetabolism in the ipsilateral PL ([18 F]FDG uptake +5.2 ± 2.3 %, p < 0.05). At 130 Hz, hypometabolism was induced in the ipsilateral PL (−2.5 ± 2.6 %, non-significant). Statistical parametric mapping revealed hypo and hypermetabolism clusters for both 60 and 130 Hz versus sham and show a certain state of alertness (increased activity in sensory and motor-related regions) mainly for 60 Hz.
Conclusions
This study suggests the potential of 60 Hz PL mPFC DBS for the treatment of disorders associated with prefrontal hypofunction.





Similar content being viewed by others
References
Pizzolato G, Mandat T (2012) Deep brain stimulation for movement disorders. Front Integr Neurosci 6:2–2
Bergey GK (2013) Neurostimulation in the treatment of epilepsy. Exp Neurol 244:87–95
Denys D, Mantione M, Figee M et al (2010) Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 67:1061
Anderson RJ, Frye MA, Abulseoud OA et al (2012) Deep brain stimulation for treatment-resistant depression: efficacy, safety and mechanisms of action. Neurosci Biobehav Rev 36:1920–1933
Kennedy SH, Giacobbe P, Rizvi SJ et al (2011) Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry 168:502–510
Howland RH, Shutt LS, Berman SR et al (2011) The emerging use of technology for the treatment of depression and other neuropsychiatric disorders. Ann Clin Psychiatry 23:48–62
Mantione M, van de Brink W, Schuurman PR, Denys D (2010) Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens. Neurosurgery 66:E218
Levy D, Shabat-Simon M, Shalev U et al (2007) Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci 27:14179–14189
Pierce RC, Vassoler FM (2013) Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology 229:487–491
Hamani C, Nobrega JN (2012) Preclinical studies modeling deep brain stimulation for depression. Biol Psychiatry 72:916–923
Lozano AM, Lipsman N (2013) Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77:406–424
Pelloux Y, Baunez C (2013) Deep brain stimulation for addiction: why the subthalamic nucleus should be favored. Curr. Opin, Neurobiol
Benabid AL, Benazzouz A, Hoffmann D et al (1998) Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord 13(Suppl 3):119–125
Lipsman N, Woodside DB, Giacobbe P et al (2013) Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet 381:1361–1370
Wyckhuys T, Raedt R, Vonck K et al (2010) Comparison of hippocampal deep brain stimulation with high (130hz) and low frequency (5hz) on afterdischarges in kindled rats. Epilepsy Res 88:239–246
Wyckhuys T, Staelens S, Van Nieuwenhuyse B et al (2010) Hippocampal deep brain stimulation induces decreased rCBF in the hippocampal formation of the rat. NeuroImage 52:55–61
Hamani C, Diwan M, Macedo CE et al (2010) Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry 67:117–124
Goddard GV, McIntyre DC, Leech CK (1969) A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol 25:295–330
Zhang Q, Wu Z-C, Yu J-T et al (2012) Anticonvulsant effect of unilateral anterior thalamic high frequency electrical stimulation on amygdala-kindled seizures in rat. Brain Res Bull 87:221–226
Montgomery EB (2010) Deep brain stimulation programming: principles and practice. Press, Oxford University
Drevets WC (2000) Neuroimaging studies of mood disorders. Biol Psychiatry 48:813–829
Chang C-C, Yu S-C, McQuoid DR et al (2011) Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res 193:1–6
Qi X-R, Kamphuis W, Wang S et al (2013) Aberrant stress hormone receptor balance in the human prefrontal cortex and hypothalamic paraventricular nucleus of depressed patients. Psychoneuroendocrinology 38:863–870
Willner P, Scheel-Krüger J, Belzung C (2013) The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 37:2331–2371
Hayashi T, Ko JH, Strafella AP, Dagher A (2013) Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci U S A 110:4422–4427
Okada K, Ota T, Iida J et al (2013) Lower prefrontal activity in adults with obsessive-compulsive disorder as measured by near-infrared spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry 43:7–13
Simmons AN, Matthews SC (2012) Neural circuitry of PTSD with or without mild traumatic brain injury: a meta-analysis. Neuropharmacology 62:598–606
Euston DR, Gruber AJ, McNaughton BL (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76:1057–1070
Miller EK, Freedman DJ, Wallis JD (2002) The prefrontal cortex: categories, concepts and cognition. Philos Trans R Soc Lond B Biol Sci 357:1123–1136
Ballard IC, Murty VP, Carter RM et al (2011) Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci 31:10340–10346
Strafella AP, Paus T, Barrett J, Dagher A (2001) Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21:RC157
Wyckhuys T, De Geeter N, Crevecoeur G et al (2013) Quantifying the effect of repetitive transcranial magnetic stimulation in the rat brain by μSPECT CBF scans. Brain Stimul 6:554–562
Parthoens J, Wyckhuys T, Stroobants S, Staelens S (2014) Small animal repetitive transcranial magnetic stimulation combined with [18 F]-FDG microPET to quantify the effect on glucose metabolism in the rat brain. submitted to Mol Imaging Biol
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Press, Academic
Deleye S, Verhaeghe J, Wyffels L et al (2014) Towards a reproducible protocol for repetitive and semi-quantitative rat brain imaging with (18) F-FDG: exemplified in a memantine pharmacological challenge. NeuroImage. doi:10.1016/j.neuroimage.2014.04.004
Bao Q, Newport D, Chen M et al (2009) Performance evaluation of the inveon dedicated pet preclinical tomograph based on the NEMA NU-4 standards. J Nucl Med 50:401–408
Schiffer WK, Mirrione MM, Dewey SL (2007) Optimizing experimental protocols for quantitative behavioral imaging with 18 F -FDG in rodents. J Nucl Med 48:277–287
Chatton J-Y, Pellerin L, Magistretti PJ (2003) GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci U S A 100:12456–12461
Hamani C, Dubiela FP, Soares JCK et al (2010) Anterior thalamus deep brain stimulation at high current impairs memory in rats. Exp Neurol 225:154–162
Smith GS (2012) Increased cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease. Arch Neurol 69:1141
Höflich A, Savli M, Comasco E et al (2013) Neuropsychiatric deep brain stimulation for translational neuroimaging. NeuroImage 79:30–41
Vitek JL (2002) Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 17:S69–S72
Volkow ND, Fowler JS, Wang G-J (2003) The addicted human brain: insights from imaging studies. J Clin Invest 111:1444–1451
Acknowledgments
This work was funded by Antwerp University, Belgium through a Ph.D. grant for J. Parthoens, a postdoctoral position for J. Verhaeghe, an associate professor position for S. Staelens and a full professor position for S. Stroobants. S. Stroobants is also supported by Antwerp University Hospital, Belgium through a departmental position. Hardware and experimental costs are supported by an IOF PoC grant (45/FA020000/27/5823) of Antwerp University. Both funding sources had no further role in the study design or in the collection, analysis, and interpretation of the data.
Conflict of Interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parthoens, J., Verhaeghe, J., Stroobants, S. et al. Deep Brain Stimulation of the Prelimbic Medial Prefrontal Cortex: Quantification of the Effect on Glucose Metabolism in the Rat Brain Using [18 F]FDG MicroPET. Mol Imaging Biol 16, 838–845 (2014). https://doi.org/10.1007/s11307-014-0757-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0757-9