Abstract
Purpose
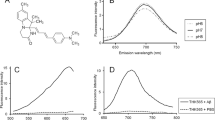
Selective visualization of amyloid-β and tau protein deposits will help to understand the pathophysiology of Alzheimer’s disease (AD). Here, we introduce a novel fluorescent probe that can distinguish between these two deposits by multispectral fluorescence imaging technique.
Procedures
Fluorescence spectral analysis was performed using AD brain sections stained with novel fluorescence compounds. Competitive binding assay using [3H]-PiB was performed to evaluate the binding affinity of BF-188 for synthetic amyloid-β (Aβ) and tau fibrils.
Results
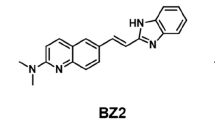
In AD brain sections, BF-188 clearly stained Aβ and tau protein deposits with different fluorescence spectra. In vitro binding assays indicated that BF-188 bound to both amyloid-β and tau fibrils with high affinity (K i < 10 nM). In addition, BF-188 showed an excellent blood–brain barrier permeability in mice.
Conclusion
Multispectral imaging with BF-188 could potentially be used for selective in vivo imaging of tau deposits as well as amyloid-β in the brain.





Similar content being viewed by others
Reference
Clinton LK, Blurton-Jones M, Myczek K et al (2010) Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci Off J Soc Neurosci 30:7281–7289
Zhou L, El-Deiry WS (2009) Multispectral fluorescence imaging. J Nucl Med Off Publ Soc Nucl Med 50:1563–1566
Ran C, Moore A (2012) Spectral unmixing imaging of wavelength-responsive fluorescent probes: an application for the real-time report of amyloid Beta species in Alzheimer’s disease. Mol Imaging Biol MIB Off Publ Acad Mol Imaging 14:293–300
Mishra R, Sjolander D, Hammarstrom P (2011) Spectroscopic characterization of diverse amyloid fibrils in vitro by the fluorescent dye Nile red. Mol Biosyst 7:1232–1240
Okamura N, Suemoto T, Furumoto S et al (2005) Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer’s disease. J Neurosci Off J Soc Neurosci 25:10857–10862
Styren SD, Hamilton RL, Styren GC, Klunk WE (2000) X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer’s disease pathology. J Histochem Cytochem Off J Histochem Soc 48:1223–1232
Velasco A, Fraser G, Delobel P et al (2008) Detection of filamentous tau inclusions by the fluorescent Congo red derivative FSB [(trans, trans)-1-fluoro-2,5-bis(3-hydroxycarbonyl-4-hydroxy)styrylbenzene]. FEBS Lett 582:901–906
Murayama H, Shin RW, Higuchi J et al (1999) Interaction of aluminum with PHFtau in Alzheimer’s disease neurofibrillary degeneration evidenced by desferrioxamine-assisted chelating autoclave method. Am J Pathol 155:877–885
Fodero-Tavoletti MT, Okamura N, Furumoto S et al (2011) (18)F-THK523: a novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 134:1089–1100
Berezin MY, Kao J, Achilefu S (2009) pH-dependent optical properties of synthetic fluorescent imidazoles. Chemistry 15:3560–3566
Kobayashi H, Ogawa M, Alford R et al (2010) New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev 110:2620–2640
Kirschner DA, Abraham C, Selkoe DJ (1986) X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-beta conformation. Proc Natl Acad Sci U S A 83:503–507
Aslund A, Sigurdson CJ, Klingstedt T et al (2009) Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem Biol 4:673–684
Grundke-Iqbal I, Iqbal K, Quinlan M et al (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Grundke-Iqbal I, Iqbal K, Tung YC et al (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 83:4913–4917
Fujiwara H, Hasegawa M, Dohmae N et al (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164
Fodero-Tavoletti MT, Mulligan RS, Okamura N et al (2009) In vitro characterisation of BF227 binding to alpha-synuclein/Lewy bodies. Eur J Pharmacol 617:54–58
Neal KL, Shakerdge NB, Hou SS, et al. (2013) Development and screening of contrast agents for in vivo imaging of Parkinson’s disease. Molecular imaging and biology: MIB: the Official pPublication of the Academy of Molecular Imaging
Berriman J, Serpell LC, Oberg KA et al (2003) Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc Natl Acad Sci U S A 100:9034–9038
Okamura N, Mori M, Furumoto S et al (2011) In vivo detection of amyloid plaques in the mouse brain using the near-infrared fluorescence probe THK-265. J Alzheimers Dis JAD 23:37–48
Acknowledgment
This study was supported by the research fund from Sumitomo Electric Industries Ltd, the Small Business Innovation Research (SBIR) program of Japan, and the Grant-in-Aid for Scientific Research on Priority Areas “Integrative Brain Research” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (20019006), and Japan Society for the Promotion of Science (JSPS).
Conflict of Interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 206 kb)
Rights and permissions
About this article
Cite this article
Harada, R., Okamura, N., Furumoto, S. et al. Use of a Benzimidazole Derivative BF-188 in Fluorescence Multispectral Imaging for Selective Visualization of Tau Protein Fibrils in the Alzheimer’s Disease Brain. Mol Imaging Biol 16, 19–27 (2014). https://doi.org/10.1007/s11307-013-0667-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0667-2