Abstract
Purpose
With single-photon emission computed tomography, simultaneous imaging of two physiological processes relies on discrimination of the energy of the emitted gamma rays, whereas the application of dual-tracer imaging to positron emission tomography (PET) imaging has been limited by the characteristic 511-keV emissions.
Procedures
To address this limitation, we developed a novel approach based on generalized factor analysis of dynamic sequences (GFADS) that exploits spatio-temporal differences between radiotracers and applied it to near-simultaneous imaging of 2-deoxy-2-[18F]fluoro-D-glucose (FDG) (brain metabolism) and 11C-raclopride (D2) with simulated human data and experimental rhesus monkey data. We show theoretically and verify by simulation and measurement that GFADS can separate FDG and raclopride measurements that are made nearly simultaneously.
Results
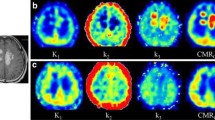
The theoretical development shows that GFADS can decompose the studies at several levels: (1) It decomposes the FDG and raclopride study so that they can be analyzed as though they were obtained separately. (2) If additional physiologic/anatomic constraints can be imposed, further decomposition is possible. (3) For the example of raclopride, specific and nonspecific binding can be determined on a pixel-by-pixel basis. We found good agreement between the estimated GFADS factors and the simulated ground truth time activity curves (TACs), and between the GFADS factor images and the corresponding ground truth activity distributions with errors less than 7.3 ± 1.3 %. Biases in estimation of specific D2 binding and relative metabolism activity were within 5.9 ± 3.6 % compared to the ground truth values. We also evaluated our approach in simultaneous dual-isotope brain PET studies in a rhesus monkey and obtained accuracy of better than 6 % in a mid-striatal volume, for striatal activity estimation.
Conclusions
Dynamic image sequences acquired following near-simultaneous injection of two PET radiopharmaceuticals can be separated into components based on the differences in the kinetics, provided their kinetic behaviors are distinct.






Similar content being viewed by others
References
Kadrmas D, Rust TC (2005) Feasibility of rapid multitracer PET tumor imaging. IEEE Trans Nuc Sci 52:1343–1347
Koeppe RA, Raffel DM, Snyder SE et al (2001) Dual-[11C]tracer single-acquisition positron emission tomography studies. J Cereb Blood Flow Metab 21:1480–1492
Converse AK, Barnhart TE, Dabbs KA et al (2004) PET measurement of rCBF in the presence of a neurochemical tracer. J Neurosci Methods 132:199–208
Kudomi N, Hayashi T, Teramoto N et al (2005) Rapid quantitative measurement of CMRO2 and CBF by dual administration of 15O-labeled oxygen and water during a single PET scan-a validation study and error analysis in anesthetized monkeys. J Cereb Blood Flow Metab 25:1209–1224
Rust TC, DiBella EVR, McGann CJ et al (2006) Rapid dual-injection single-scan 13N-ammonia PET for quantification of rest and stress myocardial blood flows. Phys Med Biol 51:5347–5362
Rust TC, Kadrmas DJ (2005) Rapid dual-tracer PTSM + ATSM PET imaging of tumour blood flow and hypoxia: a simulation study. Phys Med Biol 51:61–75
Watabe H, Ikoma Y, Kimura Y et al (2006) PET kinetics analysis—compartment model. Ann Nucl Med 20:583–588
Black NF, McJames S, Rust TC et al (2008) Evaluation of rapid dual-tracer 62Cu-PTSM + 62 Cu-ATSM PET in dogs with spontaneously occurring tumors. Phys Med Biol 53:217–232
Plotkin M, Amthauer H, Klaffke S et al (2005) Combined 123I-FP-CIT and 123I-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J Neural Transmiss 112:677–692
Knudsen GM, Karlsborg M, Thomsen G et al (2004) Imaging of dopamine transporters and D2 receptors in patients with Parkinson's disease and multiple system atrophy. Eur J Nucl Med Mol Imag 31:1631–1638
Leenders KL (2003) Significance of non-presynaptic SPECT tracer methods in Parkinson's disease. Movement Disord 18:39–42
El Fakhri G, Sitek A, Zimmerman RE, Ouyang J (2006) Generalized five dimensional dynamic and spectral factor analysis. Med Phys 33:1016–1024
Benali H, Guédon JP, Buvat I et al (1994) A statistical model for tomographic reconstruction methods using spline functions. SPIE 2299:242–251
Wu HM, Hoh CK, Buxton DB et al (1995) Quantification of myocardial blood flow using dynamic nitrogen-13-ammonia PET studies and factor analysis of dynamic structures. J Nucl Med 36:2087–2093
Kao CM, Chen CT, Wernick MN (2000) Statistical analysis of dynamic sequences for functional imaging. SPIE 3978:347–353
El Fakhri G, Sitek A, Guérin B et al (2005) Quantitative dynamic cardiac 82Rb-PET imaging using generalized factor and compartment analyses. J Nucl Med 46:1264–1271
Houston AS (1984) The effect of apex-finding errors on factor images obtained from factor analysis and oblique transformation. Phys Med Biol 29:1109–1116
Samal M, Karny M, Surova H et al (1989) On the existence of an unambiguous solution in factor analysis of dynamic studies. Phys Med Biol 34:223–228
Van Daele M, Joosten J, Devos P et al (1990) Background correction in factor analysis of dynamic scintigraphic studies: necessity and implementation. Phys Med Biol 35:1477–1485
Buvat I, Benali H, Frouin F et al (1993) Target apex-seeking in factor analysis of medical image sequences. Phys Med Biol 38:123–138
Sitek A, Di Bella EVR, Gullberg GT (2000) Factor analysis with a priori knowledge—application in dynamic cardiac SPECT. Phys Med Biol 45:2619–2638
Sitek A, Gullberg GT, Huesman RH (2002) Correction for ambiguous solutions in factor analysis using a penalized least squares objective. IEEE Trans Med Imag 21:216–225
Di Paola R, Bazin JP, Aubry F et al (1989) Handling of dynamic sequences in nuclear medicine. IEEE Trans Nucl Sci 29:1310–1321
Phelps ME, Huang SC, Hoffman EJ et al (1979) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6:371–388
Lewellen TK, Harrison RL, Vannoy S (1998) The SimSET program. In: Ljungberg M, Strand S, King MA (eds) Monte Carlo simulations in nuclear medicine. IOP Publishing, Philadelphia, pp 77–92
Guerin B, El Fakhri G (2008) Realistic PET Monte Carlo simulation with pixellated block detectors, light sharing, random coincidences and dead-time modeling. IEEE Nucl Sci 55:942–952
Farde L, Eriksson L, Blomquist G, Halldin C (1989) Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET—a comparison to the equilibrium analysis. J Cereb Blood Flow Metab 9:696–708
Endres CJ, Kolachana BS, Saunders RC et al (1997) Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab 17:932–942
Sossi V, Camborde ML, Blinder S et al (2005) Dynamic imaging on the high resolution research tomograph (HRRT): non-human primate studies. IEEE Trans Nucl Sci Med Imag Proc 2005:1981–1985
Acknowledgments
This work was supported by NIH grants R01-HL110241 and R01CA165221. Drs. El Fakhri, Trott, Sitek Alpert, and Bonab are with the Department of Radiology, Division of Nuclear Medicine and Molecular Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114.
Conflict of Interest
There are no conflict of interests/financial disclosures to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Fakhri, G., Trott, C.M., Sitek, A. et al. Dual-Tracer PET Using Generalized Factor Analysis of Dynamic Sequences. Mol Imaging Biol 15, 666–674 (2013). https://doi.org/10.1007/s11307-013-0631-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-013-0631-1