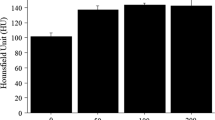
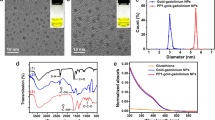
Abstract
Purpose
Inflammation and angiogenesis are important contributors to vascular disease. We evaluated imaging both of these biological processes, using Arg–Gly–Asp (RGD)-conjugated human ferritin nanoparticles (HFn), in experimental carotid and abdominal aortic aneurysm (AAA) disease.
Procedures
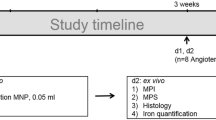
Macrophage-rich carotid lesions were induced by ligation in hyperlipidemic and diabetic FVB mice (n = 16). AAAs were induced by angiotensin II infusion in apoE−/− mice (n=10). HFn, with or without RGD peptide, was labeled with Cy5.5 and injected intravenously for near-infrared fluorescence imaging.
Results
RGD-HFn showed significantly higher signal than HFn in diseased carotids and AAAs relative to non-diseased regions, both in situ (carotid: 1.88 ± 0.30 vs. 1.17 ± 0.10, p = 0.04; AAA: 2.59 ± 0.24 vs. 1.82 ± 0.16, p = 0.03) and ex vivo. Histology showed RGD-HFn colocalized with macrophages in carotids and both macrophages and neoangiogenesis in AAA lesions.
Conclusions
RGD-HFn enhances vascular molecular imaging by targeting both vascular inflammation and angiogenesis, and allows more comprehensive detection of high-risk atherosclerotic and aneurysmal vascular diseases.






Similar content being viewed by others
References
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Galis ZS, Sukhova GK, Lark MW, Libby P (1994) Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94:2493–2503
Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P (1998) Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest 102:576–583
Shimizu K, Mitchell RN, Libby P (2006) Inflammation and cellular immune response in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 26:987–994
Lindholt JS, Vammen S, Fasting H, Henneberg EW, Heickendorff L (2000) The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur J Vasc Endovasc Surg 20:281–285
Virmani R, Kolodgie FD, Burke AP et al (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25:2054–2061
Tedesco MM, Terashima M, Blankenberg FG et al (2009) Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arterioscler Thromb Vasc Biol 29:1452–1457
Saraff K, Babamusta F, Cassis LA, Daugherty A (2003) Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused. Apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol 23:1621–1426
Paik DC, Fu C, Bhattacharya J, Tilson MD (2004) Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exper Mol Med 36:524–533
Choke E, Thompso MM, Dawson J et al (2006) Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler Thromb Vasc Biol 26:2077–2082
Hoshiga M, Alpers CE, Smith LL, Giachelli CM, Schwartz SM (1995) Alpha-v beta-3 integrin expression in normal and atherosclerotic artery. Circ Res 77:1129–1135
Antonov AS, Kolodgie FD, Munn DH, Gerrity RG (2004) Regulation of macrophage foam cell formation by alphaVbeta3 integrin: potential role in human atherosclerosis. Am J Pathol 165:247–258
Waldeck J, Häger F, Höltke C et al (2008) Fluorescence reflectance imaging of macrophage-rich atherosclerotic plaques using an alphavbeta3 integrin-targeted fluorochrome. J Nucl Med 49:1845–1851
Laitinen I, Saraste A, Weidl E et al (2009) Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging 2:331–338
Winter PM, Morawski AM, Caruthers SD et al (2003) Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha (v) beta3-integrin-targeted nanoparticles. Circulation 108:2270–2274
Liepold L, Anderson S, Willits D et al (2007) Viral capsids as MRI contrast agents. Magn Reson Med 58:871–879
Hooker JM, Datta A, Botta M, Raymond KN, Francis MB (2007) Magnetic resonance contrast agents from viral capsid shells: a comparison of exterior and interior cargo strategies. Nano Lett 7:2207–2210
Anderson EA, Isaacman S, Peabody DS, Wang EY, Canary JW, Kirshenbaum K (2006) Viral nanoparticles donning a paramagnetic coat: conjugation of MRI contrast agents to the MS2 capsid. Nano Lett 6:1160–1164
Uchida M, Terashima M, Cunningham CH et al (2008) A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn Reson Med 60:1073–1081
Uchida M, Flenniken ML, Allen M et al (2006) Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles. J Am Chem Soc 128:16626–16633
Aime S, Frullano L, Geninatti, Crich S (2002) Compartmentalization of a gadolinium complex in the apoferritin cavity: a route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew Chem Int Ed 41:1017–1019
Flenniken ML, Willits DA, Brumfield S, Young MJ, Douglas T (2003) The small heat shock protein cage from methanococcus jannaschii is a versatile nanoscale platform for genetic and chemical modification. Nano Lett 3:1573–1576
Liepold LO, Abedin MJ, Buckhouse ED, Frank JA, Young MJ, Douglas T (2009) Supramolecular protein cage composite MR contrast agents with extremely efficient relaxivity properties. Nano Lett 9:4520–4526
Terashima M, Uchida M, Kosuge H et al (2011) Human ferritin cages for imaging vascular macrophages. Biomaterials 32:1430–1437
Uchida M, Willits DA, Muller K et al (2009) Intracellular distribution of macrophage targeting ferritin–iron oxide nanocomposite. Adv Mater 21:458–462
Arap W, Pasqualini R, Ruoslahti E (1998) Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279:377–380
Kułdo JM, Ogawara KI, Asgeirsdóttir SA et al (2005) Molecular pathways of endothelial cell activation for (targeted) pharmacological intervention of chronic inflammatory diseases. Curr Vasc Pharmacol 3:11–39
Terashima M, Ehara S, Yang E et al (2010) In vivo bioluminescence imaging of inducible nitric oxide synthase gene expression in vascular inflammation. Mol Imaging Biol. doi:10.1007/s11307-010-0451-5
Kumar A, Lindner V (1997) Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 17:2238–2244
Daugherty A, Manning MW, Cassis LA (2000) Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 105:1605–1612
Pang JH, Jiang MJ, Chen YL et al (1996) Increased ferritin gene expression in atherosclerotic lesions. J Clin Invest 97:2204–2212
Li W, Xu LH, Forssell C, Sullivan JL, Yuan XM (2008) Overexpression of transferrin receptor and ferritin related to clinical symptoms and destabilization of human carotid plaques. Exp Biol Med 233:818–826
Brooks PC, Clark RA, Cheresh DA (1994) Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264:569–571
Ntziachristos V, Ripoll J, Wang LV, Weissleder R (2005) Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol 23:313–320
Nahrendorf M, Waterman P, Thurber G et al (2009) Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol 29:1444–1451
Acknowledgments
We thank Dr. Matthew Bogyo and his laboratory for their assistance with mouse fluorescence molecular tomography. We thank support from the National Institutes of Health (R21 EB005364, R01 HL078678, P50 HL083800).
Disclosures
Dr. McConnell's laboratory receives research support from GE Healthcare and he is on a scientific advisory board for Kowa, Inc. Dr. Dalman's laboratory receives AAA research support from Medtronic AVE and Carolus Pharmaceuticals. The other authors have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Bio-distribution analysis of RGD-HFn and HFn based on ex vivo Cy5.5 signal. The distribution of nanoparticles in the major organs after 48 hours was similar between RGD+ (injected with RGD-HFn-Cy5.5) and RGD− (injected with HFn-Cy5.5) groups (p = NS RGD+ vs. RGD− for all organs) (JPEG 57 kb)
Supplemental Figure 2
Macrophage immunofluorescence staining of representative non-ligated right carotid arteries in RGD− group. Immunofluorescence double staining for HFn-Cy5.5 and macrophages (stained with Mac-3) demonstrated very little macrophage infiltration and minimal Cy5.5 signal in non-diseased right carotid arteries (JPEG 42 kb)
Supplemental Figure 3
. Smooth muscle cell (SMC) immunofluorescence staining of representative ligated left carotid arteries. RGD-HFn-Cy5.5 and HFn-Cy5.5 both showed limited colocalization to carotid SMCs (see text for quantitative colocalization analysis) (JPEG 105 kb)
Supplemental Figure 4
. Smooth muscle cell (SMC) immunofluorescence staining of representative abdominal aortic aneurysms (AAA). RGD-HFn-Cy5.5 and HFn-Cy5.5 both showed limited colocalization to AAA SMCs (see text for quantitative colocalization analysis) (JPEG 88 kb)
Rights and permissions
About this article
Cite this article
Kitagawa, T., Kosuge, H., Uchida, M. et al. RGD-Conjugated Human Ferritin Nanoparticles for Imaging Vascular Inflammation and Angiogenesis in Experimental Carotid and Aortic Disease. Mol Imaging Biol 14, 315–324 (2012). https://doi.org/10.1007/s11307-011-0495-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0495-1