Abstract
Purpose
Molecular imaging using positron emission tomography (PET) radiotracers targeted to tumor vasculature offers a noninvasive method for early detection of tumor angiogenesis and efficient monitoring of response to anti-tumor vasculature therapy. The previous in vitro results demonstrated that the GX1 peptide, identified by phage display technology, is a tumor vasculature endothelium-specific ligand. In this study, we evaluated a 64Cu-labeled GX1 peptide as a potential radiotracer for microPET imaging of tumor vasculature in a U87MG tumor xenografted mouse model.
Methods
Macrocyclic chelating agent 1,4,7,10-tetraazacyclododecane-N, N′, N′′, N′′′-tetraacetic acid (DOTA)-conjugated GX1 peptide was synthesized and radiolabeled with 64Cu (t 1/2 = 12.7 h) in ammonium acetate buffer. The 64Cu-labeled GX1 peptide was then subjected to in vitro tumor cell uptake study, small animal PET and direct tissue sampling biodistribution studies in a U87MG tumor xenografted mouse model.
Results
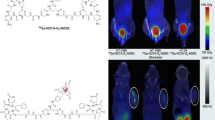
The in vitro experiment demonstrated that 64Cu-DOTA-GX1 is stable in PBS with more than 91% of 64Cu-DOTA-GX1 peptide remaining intact after 24 h of incubation. Cellular uptake and retention studies revealed 64Cu-DOTA-GX1 binds to U87MG glioma cells and has good tumor cell retention. For small animal PET imaging studies, the U87MG tumors were all clearly visible with high contrast to contralateral background at all measured time points after injection of 64Cu-DOTA-GX1 while high accumulation in liver and kidneys were also observed at early time points. The U87MG tumor uptake was determined to be the highest (7.97 ± 0.75%ID/g) at 24 h pi. The blocking experiment was achieved by co-injection of 64Cu-DOTA-GX1 with non-radiolabeled GX1 peptide (20 mg/kg) at 24 h pi, suggesting 64Cu-DOTA-GX1 is a target-specific tracer. Furthermore, the biodistribution results were consistent with the quantification of microPET imaging, demonstrating the highest ratio (16.09 ± 1.21) of tumor/muscle uptake of 64Cu-DOTA-GX1 at 24 h pi for non-blocking group and significant decreased ratio (6.57 ± 0.58) for blocking group. Finally, metabolic studies suggested that 64Cu-DOTA-GX1 is stable in mouse blood and urine in vivo at early time point while the metal transchelation may also occur in mouse liver and kidneys.
Conclusion
Our studies demonstrate that 64Cu-DOTA-GX1 is a promising radiotracer for imaging tumor vasculature.






Similar content being viewed by others
Abbreviations
- PET:
-
positron emission tomography
- HPLC:
-
high-performance liquid chromatography
- TLC:
-
thin-layer chromatography
- %ID/g:
-
percentage injected dose per gram of tissue
- pi:
-
postinjection
- GX1:
-
cyclo(CGNSNPKSC) peptide
- PBS:
-
phosphate-buffered saline
- DOTA:
-
1,4,7,10-tetraazacyclododecane-N, N′, N′′, N′′′-tetraacetic acid
- Boc:
-
t-butoxycarbonyl
- NHS:
-
N-hydroxysuccinimide
- TFA:
-
trifluoroacetic acid
- EDTA:
-
ethylenediaminetetraacetic acid
- DMF:
-
N,N-dimethylformamide
- DIPEA:
-
diisopropylethylamine
References
Weissleder R, Mahmood U (2001) Molecular imaging. Radiology 219:316–333
Chen K, Chen X (2010) Design and development of molecular imaging probes. Curr Top Med Chem 10:1227–1236
Massoud TF, Gambhir SS (2003) Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 17:545–580
Chen K, Conti PS (2010) Target-specific delivery of peptide-based probes for PET imaging. Adv Drug Deliv Rev 62:1005–1022
Deutscher SL (2010) Phage display in molecular imaging and diagnosis of cancer. Chem Rev 110:3196–3211
Chen B, Cao S, Zhang Y, Wang X, Liu J, Hui X, Wan Y, Du W, Wang L, Wu K, Fan D (2009) A novel peptide (GX1) homing to gastric cancer vasculature inhibits angiogenesis and cooperates with TNF alpha in anti-tumor therapy. BMC Cell Biol 10:63
Zhi M, Wu KC, Dong L, Hao ZM, Deng TZ, Hong L, Liang SH, Zhao PT, Qiao TD, Wang Y, Xu X, Fan DM (2004) Characterization of a specific phage-displayed peptide binding to vasculature of human gastric cancer. Cancer Biol Ther 3:1232–1235
Hui X, Han Y, Liang S, Liu Z, Liu J, Hong L, Zhao L, He L, Cao S, Chen B, Yan K, Jin B, Chai N, Wang J, Wu K, Fan D (2008) Specific targeting of the vasculature of gastric cancer by a new tumor-homing peptide CGNSNPKSC. J Control Release 131:86–93
Cai W, Gambhir SS, Chen X (2008) Chapter 7. Molecular imaging of tumor vasculature. Methods Enzymol 445:141–176
Weissleder R (2006) Molecular imaging in cancer. Science 312:1168–1171
Weissleder R (2002) Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer 2:11–18
Cai W, Rao J, Gambhir SS, Chen X (2006) How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther 5:2624–2633
Blower PJ, Lewis JS, Zweit J (1996) Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl Med Biol 23:957–980
Liu S (2008) Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv Drug Deliv Rev 60:1347–1370
Sun X, Anderson CJ (2004) Production and applications of copper-64 radiopharmaceuticals. Methods Enzymol 386:237–261
Anderson CJ, Ferdani R (2009) Copper-64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother Radiopharm 24:379–393
Shokeen M, Anderson CJ (2009) Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET). Acc Chem Res 42:832–841
Liu Z, Niu G, Wang F, Chen X (2009) (68)Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur J Nucl Med Mol Imaging 36:1483–1494
Sun X, Niu G, Yan Y, Yang M, Chen K, Ma Y, Chan N, Shen B, Chen X (2010) Phage display-derived peptides for osteosarcoma imaging. Clin Cancer Res 16:4268–4277
Chen K, Chen X (2011) PET Imaging of cancer biology: current status and future prospects. Semin Oncol 38:70–86
Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, Reinmuth N (2001) Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol 28:94–104
Kuwano M, Fukushi J, Okamoto M, Nishie A, Goto H, Ishibashi T, Ono M (2001) Angiogenesis factors. Intern Med 40:565–572
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J (2000) Vascular-specific growth factors and blood vessel formation. Nature 407:242–248
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353–364
Ueberberg S, Schneider S (2010) Phage library-screening: a powerful approach for generation of targeting-agents specific for normal pancreatic islet-cells and islet-cell carcinoma in vivo. Regul Pept 160:1–8
Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari S, Verma IM (2011) Feature article: transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci U S A (in press)
Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, Conti PS (2004) microPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med 45:1390–1397
Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, Gambhir SS, Chen X (2005) microPET imaging of glioma integrin {alpha}v{beta}3 expression using (64)Cu-labeled tetrameric RGD peptide. J Nucl Med 46:1707–1718
Li ZB, Cai W, Cao Q, Chen K, Wu Z, He L, Chen X (2007) (64)Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor alpha(v)beta(3) integrin expression. J Nucl Med 48:1162–1171
Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X (2006) In vitro and in vivo characterization of 64Cu-labeled Abegrin, a humanized monoclonal antibody against integrin alpha v beta 3. Cancer Res 66:9673–9681
Chen X, Liu S, Hou Y, Tohme M, Park R, Bading JR, Conti PS (2004) MicroPET imaging of breast cancer alphav-integrin expression with 64Cu-labeled dimeric RGD peptides. Mol Imaging Biol 6:350–359
Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, Anderson CJ (2004) Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem 47:1465–1474
Sun X, Wuest M, Weisman GR, Wong EH, Reed DP, Boswell CA, Motekaitis R, Martell AE, Welch MJ, Anderson CJ (2002) Radiolabeling and in vivo behavior of copper-64-labeled cross-bridged cyclam ligands. J Med Chem 45:469–477
Cai H, Li Z, Huang CW, Shahinian AH, Wang H, Park R, Conti PS (2010) Evaluation of copper-64 labeled AmBaSar conjugated cyclic RGD peptide for improved MicroPET imaging of integrin alphavbeta3 expression. Bioconjug Chem 21:1417–1424
Cai H, Li Z, Huang CW, Park R, Shahinian AH, Conti PS (2010) An improved synthesis and biological evaluation of a new cage-like bifunctional chelator, 4-((8-amino-3, 6, 10, 13, 16, 19-hexaazabicyclo[6.6.6]icosane-1-ylamino)methyl)benzoic acid, for 64Cu radiopharmaceuticals. Nucl Med Biol 37:57–65
Chen X (2006) Multimodality imaging of tumor integrin alphavbeta3 expression. Mini Rev Med Chem 6:227–234
Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M (2001) Noninvasive imaging of alpha(v)beta3 integrin expression using 18 F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res 61:1781–1785
Shi J, Kim YS, Zhai S, Liu Z, Chen X, Liu S (2009) Improving tumor uptake and pharmacokinetics of (64)Cu-labeled cyclic RGD peptide dimers with Gly(3) and PEG(4) linkers. Bioconjug Chem 20:750–759
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health, the USC Department of Radiology, and the Provost’s Biomedical Imaging Science Initiative.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, K., Sun, X., Niu, G. et al. Evaluation of 64Cu Labeled GX1: A Phage Display Peptide Probe for PET Imaging of Tumor Vasculature. Mol Imaging Biol 14, 96–105 (2012). https://doi.org/10.1007/s11307-011-0479-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0479-1