Abstract
Purpose
The purpose of this study is to evaluate the time course of early chemotherapy response in patients with aggressive non-Hodgkin's lymphoma (NHL) by magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT).
Procedures
Eight patients with histologically proven aggressive NHL were imaged by MRI and PET/CT before treatment (E1), 1 week (E2), and two cycles (E3) after chemotherapy.
Results
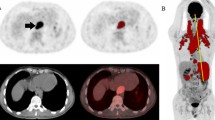
The mean tumor volume on MRI was 276 mL at baseline; it decreased 58% at E2 (p < 0.05) and 65% further at E3 (p < 0.05), giving a total decrease of 84% (p < 0.05). All the imaged pre-therapy tumors were strongly positive on PET/CT, with a mean maximum standardized uptake value (SUVmax) of 20. The SUVmax decreased 60% at E2 (p < 0.05) and 59% further at E3 (p < 0.05), giving a total decrease of 83% (p < 0.05). The active tumor burden (mean 229 mL) decreased 66% at E2 (p < 0.05). The tumor volume on MRI correlated with the active tumor volume on fused PET/CT images in the same region of interest at both E1 and E2 (r = 0.88, p < 0.01, respectively).
Conclusions
Standard chemotherapy causes rapid decrease of both tumor metabolic activity and volume as early as 1 week, which continues to decline during therapy. Both volumetric MRI and PET/CT are valuable tools for early treatment response evaluation of aggressive NHL.



Similar content being viewed by others
References
Cheson BD, Horning SJ, Coiffier B et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244–1253
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Schoder H, Noy A, Gonen M et al (2005) Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol 23:4643–4651
Spaepen K, Stroobants S, Dupont P et al (2002) Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol 13:1356–1363
Ngeow JY, Quek RH, Ng DC et al (2009) High SUV uptake on FDG-PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol 20:1543–1547
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Therasse P, Eisenhauer EA, Verweij J (2006) RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 42:1031–1039
Rossi M, Dastidar P, Pertovaara H et al (2009) Response analysis of non-hodgkin lymphoma using magnetic resonance imaging-based volumes. J Comput Assist Tomogr 33:466–474
Ghanem N, Lohrmann C, Engelhardt M et al (2006) Whole-body MRI in the detection of bone marrow infiltration in patients with plasma cell neoplasms in comparison to the radiological skeletal survey. Eur Radiol 16:1005–1014
Schmidt GP, Baur-Melnyk A, Herzog P et al (2005) High-resolution whole-body magnetic resonance image tumor staging with the use of parallel imaging versus dual-modality positron emission tomography-computed tomography: experience on a 32-channel system. Invest Radiol 40:743–753
Antoch G, Vogt FM, Freudenberg LS et al (2003) Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. Jama 290:3199–3206
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Heinonen T, Dastidar P, Kauppinen P, Malmivuo J, Eskola H (1998) Semi-automatic tool for segmentation and volumetric analysis of medical images. Med Biol Eng Comput 36:291–296
Heinonen T, Dastidar P, Eskola H, Frey H, Ryymin P, Laasonen E (1998) Applicability of semi-automatic segmentation for volumetric analysis of brain lesions. J Med Eng Technol 22:173–178
Dastidar P, Heinonen T, Vahvelainen T, Elovaara I, Eskola H (1999) Computerised volumetric analysis of lesions in multiple sclerosis using new semi-automatic segmentation software. Med Biol Eng Comput 37:104–107
Saarinen T, Dastidar P, Peltola R et al (2005) Evaluation of the treatment outcome of lymphoma patients after the first treatment using magnetic resonance imaging based volumetry. The 3 rd European Medical and Biological Engineering Conference EMBEC 05, Prague, Czech Republik. IFMBE Proceedings. Vol. 11
(1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329:987–994
Cazaentre T, Morschhauser F, Vermandel M et al (2010) Pre-therapy 18F-FDG PET quantitative parameters help in predicting the response to radioimmunotherapy in non-Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 37:494–504
Haioun C, Itti E, Rahmouni A et al (2005) [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 106:1376–1381
Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR (2005) FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol 16:1514–1523
Yamane T, Daimaru O, Ito S et al (2004) Decreased 18F-FDG uptake 1 day after initiation of chemotherapy for malignant lymphomas. J Nucl Med 45:1838–1842
Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ (2002) PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin’s disease. J Nucl Med 43:1018–1027
Torizuka T, Nakamura F, Kanno T et al (2004) Early therapy monitoring with FDG-PET in aggressive non-Hodgkin’s lymphoma and Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging 31:22–28
Romer W, Hanauske AR, Ziegler S et al (1998) Positron emission tomography in non-Hodgkin’s lymphoma: assessment of chemotherapy with fluorodeoxyglucose. Blood 91:4464–4471
Schaefer NG, Strobel K, Taverna C, Hany TF (2007) Bone involvement in patients with lymphoma: the role of FDG-PET/CT. Eur J Nucl Med Mol Imaging 34:60–67
Moulin-Romsee G, Hindie E, Cuenca X et al (2010) (18)F-FDG PET/CT bone/bone marrow findings in Hodgkin’s lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging 37:1095–1105
Schmidt GP, Reiser MF, Baur-Melnyk A (2009) Whole-body MRI for the staging and follow-up of patients with metastasis. Eur J Radiol 70:393–400
Acknowledgements
This study was supported by the Biomedical Image Quantification/University Alliance Finland and the Elna Savolainen Fund.
Conflict of interest
We have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, X., Dastidar, P., Pertovaara, H. et al. Early Treatment Response Evaluation in Patients with Diffuse Large B-Cell Lymphoma—A Pilot Study Comparing Volumetric MRI and PET/CT. Mol Imaging Biol 13, 785–792 (2011). https://doi.org/10.1007/s11307-010-0404-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0404-z