Abstract
Materials and Methods
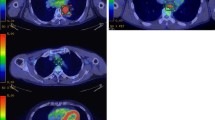
We investigated the utility of metabolic tumor width parameters in predicting response to chemoradiotherapy and in predicting disease-free survival in patients with esophageal cancer. Furthermore, we evaluated the possible confounding effect of therapy-induced esophagitis on the evaluation of treatment response. Forty-nine patients with squamous cell carcinoma, who had undergone positron emission tomography/computed tomography (PET/CT) exams before and after neoadjuvant chemoradiotherapy, were included in the study. In the slice with the maximum 2-deoxy-2-[F-18]fluoro-d-glucose (FDG) uptake of the tumor, the following metabolic tumor width parameters were measured: Area of the tumor, maximum diameter of the tumor, maximum and mean standardized uptake value (SUV). Furthermore, the “diameter-SUV index” was calculated by multiplying the tumor diameter by the mean SUV.
Results
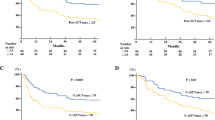
The decrease of the metabolic tumor diameter between pre- and post-treatment PET/CT scans was the single best predictor of treatment response and tumor-free survival. However, the accuracy of predicting response and survival was even higher when using the decrease of the “diameter-SUV index” as the metabolic criterion for treatment response. A decrease by more than 55% of the diameter-SUV index identified pathologic responders (n = 22) with a sensitivity of 91% and a specificity of 93%. Radiation esophagitis was found to have a significant impact on the assessment of treatment response when evaluating therapy response based on the maximum SUV, whereas no confounding effect of radiation esophagitis was seen when evaluating therapy response based on the tumor diameter or the diameter-SUV index.
Conclusion
The present study shows that tumor width parameters, especially the tumor diameter or the combination of diameter and SUV in the “diameter-SUV index”, are valuable for predicting tumor-free survival and treatment response independent from the presence of radiation esophagitis.



Similar content being viewed by others
References
Bar-Shalom R, Guralnik L, Tsalic M et al (2005) The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging 32:918–924
Yuan S, Yu Y, Chao KS et al (2006) Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med 47:1255–1259
Swisher SG, Maish M, Erasmus JJ et al (2004) Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 78:1152–1160
Wieder HA, Beer AJ, Lordick F et al (2005) Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med 46:2029–2034
Lordick F, Ott K, Krause BJ et al (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8:797–805
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 80:11–20
Medical Research Council Oesophageal Cancer Working Party (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359:1727–1733
Kelsen DP, Ginsberg R, Pajak TF et al (1998) Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339:1979–1984
Brücher BL, Weber W, Bauer M et al (2001) Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg 233:300–309
Song SY, Kim JH, Ryu JS et al (2005) FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys 63:1053–1059
Brink I, Hentschel M, Bley TA et al (2004) Effects of neoadjuvant radio-chemotherapy on 18F-FDG-PET in esophageal carcinoma. Eur J Surg Oncol 30:544–550
Ott K, Weber W, Siewert JR (2006) The importance of PET in the diagnosis and response evaluation of esophageal cancer. Dis Esophagus 19:433–442
Ott K, Weber WA, Lordick F et al (2006) Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 24:4692–4698
Swisher SG, Maish M, Erasmus JJ et al (2004) Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 78:1152–1160
Flamen P, Van Cutsem E, Lerut A et al (2002) Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol 13:361–368
Wieder HA, Brücher BL, Zimmerman F et al (2004) Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol 22:900–908
Rizk N, Downey RJ, Akhurst T et al (2006) Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg 81:1076–1081
Blackstock AW, Farmer MR, Lovato J et al (2006) A prospective evaluation of the impact of 18-F-fluoro-deoxy-D-glucose positron emission tomography staging on survival for patients with locally advanced esophageal cancer. Int J Radiat Oncol Biol Phys 64:455–460
Cerfolio RJ, Bryant AS (2006) Maximum standardized uptake values on positron emission tomography of esophageal cancer predicts stage, tumor biology, and survival. Ann Thorac Surg 82:391–394
Song SY, Kim JH, Ryu JS et al (2005) FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys 63:1053–1059
Larson SM (1994) Cancer or inflammation? A holy grail for nuclear medicine. J Nucl Med 35:1653–1655
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roedl, J.B., Halpern, E.F., Colen, R.R. et al. Metabolic Tumor Width Parameters as Determined on PET/CT Predict Disease-free Survival and Treatment Response in Squamous Cell Carcinoma of the Esophagus. Mol Imaging Biol 11, 54–60 (2009). https://doi.org/10.1007/s11307-008-0169-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0169-9