Abstract
Purpose
The aim of the study is to compare the tumor-specific targeting, pharmacokinetics, and biodistribution of 64Cu-DOTA-HB22.7 when administered to xenograft-bearing mice intravenously (IV), intraperitoneally (IP), and subcutaneously (SQ).
Procedures
Mice bearing human non-Hodgkin’s lymphoma (NHL) xenografts were injected IV, IP, or SQ with 64Cu-DOTA-HB22.7. Xenograft targeting was evaluated by micro positron emission tomography (microPET) and confirmed by organ biodistribution studies. Blood measurements of 64Cu were performed to determine the pharmacokinetics and clearance of 64Cu-DOTA-HB22.7.
Results
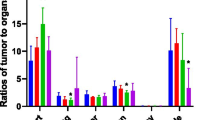
64Cu-DOTA-HB22.7 demonstrated equivalent tumor targeting within 24–48 h, regardless of the route of administration. Organ biodistribution confirmed tumor-specific targeting. Blood pharmacokinetics demonstrated that 64Cu-DOTA-HB22.7 accessed the bloodstream after IP and SQ administration to a similar degree as IV administration, albeit at a slower rate.
Conclusions
These findings establish 64Cu-DOTA-HB22.7 as a potential radioimmunotherapeutic and/or NHL-specific imaging agent. These findings provide evidence that IP and SQ administration can achieve results equivalent to IV administration and may lead to more efficient, reproducible treatment plans for antibody-based therapeutics.





Similar content being viewed by others
Abbreviations
- 64Cu:
-
Copper-64
- DOTA-NHS-ester:
-
1,4,7,10-tetraazacyclododecane-N,N′,N′′,N′′′-tetraacetic acid mono(N-hydroxysuccinimidyl ester)
- Ig:
-
immunoglobulin
- IV:
-
intravenous
- IP:
-
intraperitoneal
- mAb:
-
monoclonal antibody
- NHL:
-
non-Hodgkin’s lymphoma
- RIT:
-
radioimmunotherapy
- SQ:
-
subcutaneous
- TLC:
-
thin layer chromatography
References
Schumer ST, Joyce RM (2003) Radioimmunotherapy for Non-Hodgkin’s Lymphoma. Progress in Oncology 46–72
Lindley C (1991) The lymphomas: Hodgkin’s disease and non-Hodgkin’s lymphomas. Am Pharm NS31 46–51
DeNardo GL (2005) Treatment of non-Hodgkin’s lymphoma (NHL) with radiolabeled antibodies (mAbs). Semin Nucl Med 35:202–211
DeNardo GL, O'Donnell RT, DeNardo SJ (2001) Radiolabeled anti-lymphoma antibodies. Cancer Chemother Biol Response Modif 19:297–308
Kukis DL, DeNardo GL, DeNardo SJ et al (1995) Effect of the extent of chelate substitution on the immunoreactivity and biodistribution of 2IT-BAT-Lym-1 immunoconjugates. Cancer Res 55:878–884
Juweid ME (2002) Radioimmunotherapy of B-cell non-Hodgkin’s lymphoma: from clinical trials to clinical practice. J Nucl Med 43:1507–1529
Riley MB, Gordon LI (2004) Efficacy and safety of radioimmunotherapy with yttrium 90 ibritumomab tiuxetan (Zevalin). Semin Oncol Nurs 20:8–13
Hjortland GO, Garman-Vik SS, Juell S (2004) Immunotoxin treatment targeted to the high-molecular-weight melanoma-associated antigen prolonging the survival of immunodeficient rats with invasive intracranial human glioblastoma multiforme. J Neurosurg 100:320–327
Kawakami K, Nakajima O, Morishita R, Nagai R (2006) Targeted anticancer immunotoxins and cytotoxic agents with direct killing moieties. Scientific World Journal 6:781–790
Tedder TF, Tuscano J, Sato S, Kehrl JH (1997) CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol 15:481–504
Sato S, Tuscano JM, Inaoki M, Tedder TF (1998) CD22 negatively and positively regulates signal transduction through the B lymphocyte antigen receptor. Semin Immunol 10:287–297
Tuscano JM, Riva A, Toscano SN, Tedder TF, Kehrl JH (1999) CD22 cross-linking generates B-cell antigen receptor-independent signals that activate the JNK/SAPK signaling cascade. Blood 94:1382–1392
Tuscano JM, O'Donnell RT, Miers LA et al (2003) Anti-CD22 ligand-blocking antibody HB22.7 has independent lymphomacidal properties and augments the efficacy of 90Y-DOTA-peptide-Lym-1 in lymphoma xenografts. Blood 101:3641–3647
Koppe MJ, Postema EJ, Aarts F, Oyen WJ, Bleichrodt RP, Boerman OC (2005) Antibody-guided radiation therapy of cancer. Cancer Metastasis Rev 24:539–567
Postema EJ, Boerman OC, Oyen WJ, Raemaekers JM, Corstens FH (2001) Radioimmunotherapy of B-cell non-Hodgkin’s lymphoma. Eur J Nucl Med 28:1725–1735
O'Grady LF, DeNardo G, DeNardo S (1986) Radiolabelled monoclonal antibodies for the detection of cancer. Am J Physiol Imaging 1:44–53
Rao AV, Akabani G, Rizzieri DA (2005) Radioimmunotherapy for Non-Hodgkin’s Lymphoma. Clin Med Res 3:157–165
Smith SV (2004) Molecular imaging with copper-64. J Inorg Biochem 98:1874–1901
Phelps ME (2000) Inaugural article: positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci USA 97:9226–9233
Winnard P Jr., Raman V (2003) Real time non-invasive imaging of receptor-ligand interactions in vivo. J Cell Biochem 90:454–463
Hale G, Rebello P, Brettman LR et al (2004) Blood concentrations of alemtuzumab and antiglobin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood 104:948–955
Meares CF, McCall MJ, Reardan DT, Goodwin DA, Diamanti CI, McTigue M (1984) Conjugation of antibodies with bifunctional chelating agents: isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Anal Biochem 142:68–78
McCarthy DW, Shefer RE, Klinkowstein RE et al (1997) Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 24:35–43
Tai YC, Chatziioannou AF, Yang Y et al (2003) MicroPET II: design, development and initial performance of an improved microPET scanner for small-animal imaging. Phys Med Biol 48:1519–1537
Chatziioannou A, Tai YC, Doshi N, Cherry SR (2001) Detector development for microPET II: a 1 microl resolution PET scanner for small animal imaging. Phys Med Biol 46:2899–2910
Qi J, Leahy RM, Cherry SR, Chatziioannou A, Farquhar TH (1998) High-resolution 3D Bayesian image reconstruction using the microPET small-animal scanner. Phys Med Biol 43:1001–1013
Chatziioannou A, Qi J, Moore A et al (2000) Comparison of 3-D maximum a posteriori and filtered backprojection algorithms for high-resolution animal imaging with microPET. IEEE Trans Med Imaging 19:507–512
Abbey CK, Borowsky AD, McGoldrick ET et al (2004) In vivo positron-emission tomography imaging of progression and transformation in a mouse model of mammary neoplasia. Proc Natl Acad Sci USA 101:11438–11443
Qi J, Leahy RM (1999) A theoretical study of the contrast recovery and variance of MAP reconstructions from PET data. IEEE Trans Med Imaging 18:293–305
Love C, Tomas MB, Tronco GG, Palestro CJ (2005) FDG PET of infection and inflammation. Radiographics 25:1357–1368
Rosenbaum SJ, Lind T, Antoch G, Bockisch A (2006) False-positive FDG PET uptake—the role of PET/CT. Eur Radiol 16:1054–1065
Jacene HA, Stearns V, Wahl RL (2006) Lymphadenopathy resulting from acute hepatitis C infection mimicking metastatic breast carcinoma on FDG PET/CT. Clin Nucl Med 31:379–381
Engel P, Wagner N, Miller AS, Tedder TF (1995) Identification of the ligand binding domains of CD22, a member of the immunoglobulin superfamily that uniquely binds a sialic acid-dependent ligand. J Exp Med 181:1581–1586
Eischen A, Doclos B, Schmitt-Goguel M et al (1994) Human resident peritoneal macrophages: phenotype and biology. Br J Haematol 88:712–722
Whitelaw DM (1966) The intravascular lifespan of monocytes. Blood 28:455–464
Garner B, van Reyk D, Dean RT, Jessup W (1997) Direct copper reduction by macrophagesIts role in low density lipoprotein oxidation. J Biol Chem 272:6927–6935
Ehrenwald E, Chisolm GM, Fox PL (1994) Intact human ceruloplasmin oxidatively modifies low density lipoprotein. J Clin Invest 93:1493–1501
Ehrenwald E, Fox PL (1996) Role of endogenous ceruloplasmin in low density lipoprotein oxidation by human U937 monocytic cells. J Clin Invest 97:884–890
Chung J, Haile DJ, Wessling-Resnick M (2004) Copper-induced ferroportin-1 expression in J774 macrophages is associated with increased iron efflux. Proc Natl Acad Sci USA 101:2700–2705
Rossi F (1986) The O2 −-forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta 853:65–89
Babior BM, Kipnes RS, Curnutte JT (1973) Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52:741–744
Pietarinen-Runtti P, Lakari E, Raivio KO, Kinnula VL (2000) Expression of antioxidant enzymes in human inflammatory cells. Am J Physiol Cell Physiol 278:C118–C125
Grunberg J, Novak-Hofer I, Honer M et al (2005) In-vivo evaluation of 177Lu- and 67/64Cu-labeled recombinant fragments of antibody chCE7 for radioimmunotherapy and PET imaging of L1-CAM positive tumors. Clin Cancer Res 11:5112–5120
Lewis MR, Boswell CA, Laforest R et al (2001) Conjugation of monoclonal antibodies with TETA using activated esters: biological comparison of 64Cu-TETA-1A3 with 64Cu-BAT-2IT-1A3. Cancer Biother Radiopharm 16:483–494
Cai W, Ebrahimnejad A, Chen K et al (2007) Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur J Nucl Med Mol Imaging 34:2024–2036
DeNardo GL, DeNardo SJ, Meares CF et al (1991) Pharmacokinetics of copper-67 conjugated Lym-1, a potential therapeutic radioimmunoconjugate, in mice and in patients with lymphoma. Antibody Immunoconj Radiopharm 4:777–785
Deshpande SV, DeNardo SJ, Meares CF et al (1988) Copper-67-labeled monoclonal antibody Lym-1, a potential radiopharmaceutical for cancer therapy: labeling and biodistribution in Raji tumored mice. J Nucl Med 29:217–225
Acknowledgements
The authors would like to thank the CMGI staff, particularly Jennifer Fung and Chris Griesemer, for their support and expertise in small animal imaging. The authors would also like to thank Yunpeng Ma for assistance with organ harvesting for biodistribution studies. The production of Cu-64 at Washington University School of Medicine is supported by the NCI grant R24 CA86307. This work was supported by R33 CA89706 (NIH, NCI); 00-00764V-0133, the University of California Cancer Research Program, VA Merit grants, 1337754 and 7855423, Small Animal Resource Program Grant R24 CA110804 Leukemia and Lymphoma Society Translational Research Award, Schwedler Foundation and the deLeuze Endowment for the non-toxic cure of lymphoma.
Author information
Authors and Affiliations
Corresponding author
Additional information
Julie L. Sutcliffe and Joseph M. Tuscano—these authors jointly supervised this study.
Rights and permissions
About this article
Cite this article
Martin, S.M., O’Donnell, R.T., Kukis, D.L. et al. Imaging and Pharmacokinetics of 64Cu-DOTA-HB22.7 Administered by Intravenous, Intraperitoneal, or Subcutaneous Injection to Mice Bearing Non-Hodgkin’s Lymphoma Xenografts. Mol Imaging Biol 11, 79–87 (2009). https://doi.org/10.1007/s11307-008-0148-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0148-1