Abstract
Purpose
Magnetic resonance imaging (MRI) can track labeled cells in the brain. The use of hemagglutinating virus of Japan envelopes (HVJ-Es) to effectively introduce the contrast agent to neural progenitor cells (NPCs) is limited to date despite their high NPC affinity.
Procedures
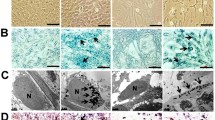
HVJ-Es and Lipofectamine 2000 were compared as transfection vehicles of superparamagnetic iron oxide (SPIO). Labeled NPCs were examined for iron content, MRI signal change, and fundamental cell characteristics. Prussian Blue staining was used after differentiation to determine SPIO localization.
Results
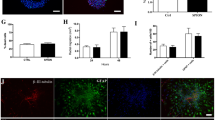
HVJ-Es transfected up to 12.5 ± 8.8 times more SPIO into NPCs. HVJ-Es do not affect cell viability or differentiation capability. Superparamagnetic iron oxide was disseminated in both the soma and neurites.
Conclusions
These findings indicate that HVJ-Es are an effective vehicle for SPIO transfection of NPCs. The intracellular localization after differentiation raises the question as to the capability of MRI to distinguish cell migration from axonal or dendritic growth in vivo.







Similar content being viewed by others
References
Le Belle JE, Svendsen CN (2002) Stem cells for neurodegenerative disorders: where can we go from here? BioDrugs 16:389–401
Lewin M, Carlesso N, Tung CH, et al. (2000) Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol 18:410–414
Bulte JW, Douglas T, Witwer B, et al. (2001) Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol 19:1141–1147
Hinds KA, Hill JM, Shapiro EM, et al. (2003) Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood 102:867–872
Modo M, Cash D, Mellodew K, et al. (2002) Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. NeuroImage 17:803–811
Van den Bos EJ, Wagner A, Mahrholdt H, et al. (2003) Improved efficacy of stem cell labeling for magnetic resonance imaging studies by the use of cationic liposomes. Cell Transplant 12:743–756
Arbab AS, Bashaw LA, Miller BR, et al. (2003) Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation 76:1123–1130
Hoehn M, Kustermann E, Blunk J, et al. (2002) Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A 99:16267–16272
Dokka S, Toledo D, Shi X, et al. (2000) Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res 17:521–525
Shimamura M, Morishita R, Endoh M, et al. (2003) HVJ-envelope vector for gene transfer into central nervous system. Biochem Biophys Res Commun 300:464–471
Popa LM, Repanovici R, Mihalache O, et al. (1982) EPR and Mossbauer spectroscopy investigations on the metal ion contents of Sedai virus components. Virologie 33:271–278
Ardizzoni SC, Michaels A, Arendash GW (1988) Labeling of neural cells by gold-filled Sendai virus envelopes before intracerebral transplantation. Science 239:635–637
Hawrylak N, Ghosh P, Broadus J, et al. (1993) Nuclear magnetic resonance (NMR) imaging of iron oxide-labeled neural transplants. Exp Neurol 121:181–192
Toyoda K, Tooyama I, Kato M, et al. (2004) Effective magnetic labeling of transplanted cells with HVJ-E for magnetic resonance imaging. NeuroReport 15:589–593
Vale RD, Hosang M, Shooter EM (1985) Sialic acid residues on NGF receptors on PC12 cells. Dev Neurosci 7:55–64
Otsu N (1979) A threshold selection method from gray-level histogram. IEEE Trans Syst Man Cybern 8:62–66
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Billotey C, Wilhelm C, Devaud M, et al. (2003) Cell internalization of anionic maghemite nanoparticles: quantitative effect on magnetic resonance imaging. Magn Reson Med 49:646–654
Kott JN, Westrum LE, Ardizzoni SC, et al. (1991) Ultrastructural localization of gold particles within neural grafts labeled with gold-filled Sendai viral envelopes. J Electron Microsc Tech 18:197–202
Acknowledgements
This work was supported by NIH/NCRR P51 RR000166 and in part by funding from the Natural Science and Engineering Research Council of Canada. The authors would like to thank Berlex Laboratories (Wayne, NJ) for their donation of Feridex IV and Raja Atallah from the Environmental Health Laboratory for his help with the atomic absorption spectrophotometry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyoshi, S., Flexman, J.A., Cross, D.J. et al. Transfection of Neuroprogenitor Cells with Iron Nanoparticles for Magnetic Resonance Imaging Tracking: Cell Viability, Differentiation, and Intracellular Localization. Mol Imaging Biol 7, 286–295 (2005). https://doi.org/10.1007/s11307-005-0008-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-005-0008-1