Abstract
Introduction
With chronic kidney disease (CKD), kidney becomes damaged overtime and fails to clean blood. Around 15% of US adults have CKD and nine in ten adults with CKD do not know they have it.
Objective
Early prediction and accurate monitoring of CKD may improve care and decrease the frequent progression to end-stage renal disease. There is an urgent demand to discover specific biomarkers that allow for monitoring of early-stage CKD, and response to treatment.
Method
To discover such biomarkers, shotgun high throughput was applied to the detection of serum metabolites biomarker discovery for early stages of CKD from 703 participants. Ultra performance liquid chromatography coupled with high-definition mass spectrometry (UPLC-HDMS)-based metabolomics was used for the determination of 703 fasting serum samples from five stages of CKD patients and age-matched healthy controls.
Results and conclusion
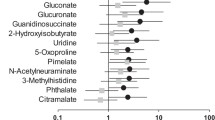
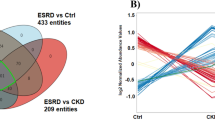
We discovered a set of metabolite biomarkers using a series of classic and neural network based machine learning techniques. This set of metabolites can separate early CKD stage patents from normal subjects with high accuracy. Our study illustrates the power of machine learning methods in metabolite biomarker study.



Similar content being viewed by others
References
Allison, D. (2017). Global metabolomics. Nature Methods, 14, 32–32. https://doi.org/10.1038/nmeth.4112.
Beckham, C., & Pal, C. (2016). A simple squared-error reformulation for ordinal classification. Paper presented at the 29th Conference on Neural Information Processing Systems (NIPS 2016), Barcelona.
Chen, H., et al. (2016). Metabolomics insights into activated redox signaling and lipid metabolism dysfunction in chronic kidney disease progression. Redox Biology, 10, 168–178.
Chen, D. Q., et al. (2017a). Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/beta-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biology, 12, 505–521. https://doi.org/10.1016/j.redox.2017.03.017.
Chen, D. Q., et al. (2017b). The link between phenotype and fatty acid metabolism in advanced chronic kidney disease. Nephrology, Dialysis, Transplantation. https://doi.org/10.1093/ndt/gfw415.
Chen, H., et al. (2017c). Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. Journal of Proteome Research, 16, 1566–1578. https://doi.org/10.1021/acs.jproteome.6b00956.
Coresh, J., et al. (2007). Prevalence of chronic kidney disease in the United States. JAMA, 298, 2038–2047. https://doi.org/10.1001/jama.298.17.2038.
Foundation, N. K. (2002). K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. American Journal of Kidney Diseases, 39, S1–S266.
Fouque, D., et al. (2006). Relationship between serum carnitine, acylcarnitines, and renal function in patients with chronic renal disease. Journal of Renal Nutrition, 16, 125–131.
Goek, O. N., et al. (2012). Serum metabolite concentrations and decreased GFR in the general population. American Journal of Kidney Diseases, 60, 197–206.
Goek, O. N., et al. (2013). Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrology, Dialysis, Transplantation, 28, 32131–32138.
Johnson, C. H., Ivanisevic, J., & Siuzdak, G. (2016). Metabolomics: Beyond biomarkers and towards mechanisms. Nature Reviews Molecular Cell Biology, 17, 451–459.
Kalim, S., & Rhee, E. P. (2017). An overview of renal metabolomics. Kidney International, 91, 61–69.
Levey, A. S., Bosch, J. P., Lewis, J. B., Greene, T., Rogers, N., & Roth, D. (1999). A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of Internal Medicine, 130, 461–470.
McQueen, R. B., Farahbakhshian, S., Bell, K. F., Nair, K. V., & Saseen, J. J. (2017). Economic burden of comorbid chronic kidney disease and diabetes. Journal of Medical Economics, 20, 585–591. https://doi.org/10.1080/13696998.2017.1288127.
Mulders, P. F. (2013). From genes to metabolomics in renal cell carcinoma translational research. European Urology, 63, 252–253.
Nicholson, J. K., & Lindon, J. C. (2008). Systems biology: Metabonomics. Nature, 455, 1054–1056. https://doi.org/10.1038/4551054a.
Qi, S., Ouyang, X., Wang, L., Peng, W., Wen, J., & Dai, Y. (2012). A pilot metabolic profiling study in serum of patients with chronic kidney disease based on (1) H-NMR-spectroscopy. Clinical and Translational Science, 5, 379–385.
Rhee, E. P., et al. (2013). A combined epidemiologic and metabolomic approach improves CKD prediction. Journal of the American Society of Nephrology, 24, 1330–1338. https://doi.org/10.1681/ASN.2012101006.
Saunders, M. R., Cifu, A., & Vela, M. (2015). Screening for Chronic Kidney Disease. JAMA-Journal of the American Medical Association, 314, 615–616. https://doi.org/10.1001/jama.2015.9425.
Sekula, P., et al. (2016). A metabolome-wide association study of kidney function and disease in the general population. Journal of the American Society of Nephrology, 27, 1175–1188. https://doi.org/10.1681/ASN.2014111099.
Tibshirani, R. (1996). Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society Series B-Methodological, 58, 267–288.
Tieleman, T., & Hinton, G. (2012). Lecture 6.5-rmsprop: Divide the gradient by a running average of its recent magnitude. COURSERA: Neural Networks for Machine Learning, 4, 26–31.
Tin Kam, H. (1998). The random subspace method for constructing decision forests. IEEE Transactions on Pattern Analysis and Machine Intelligence, 20, 832–844. https://doi.org/10.1109/34.709601.
USRDS (2013). USRDS 2013 Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. https://www.usrds.org/atlas13.aspx.
Venables, W. N., & Ripley, B. D. (2002). Modern applied statistics with S (4th ed.). New York: Springer.
Vupputuri, S., et al. (2014). The economic burden of progressive chronic kidney disease among patients with type 2 diabetes. Journal of Diabetes and its Complications, 28, 10–16. https://doi.org/10.1016/j.jdiacomp.2013.09.014.
Wang, H. S., Li, G. D., & Tsai, C. L. (2007). Regression coefficient and autoregressive order shrinkage and selection via the lasso. Journal of the Royal Statistical Society Series B-Statistical Methodology, 69, 63–78.
Wang, V., Vilme, H., Maciejewski, M. L., & Boulware, L. E. (2016). The economic burden of chronic kidney disease and end-stage renal disease. Seminars in Nephrology, 36, 319–330. https://doi.org/10.1016/j.semnephrol.2016.05.008.
Want, E. J., et al. (2010). Global metabolic profiling procedures for urine using UPLC-MS. Nature Protocols, 5, 1005–1018.
Want, E. J., et al. (2013). Global metabolic profiling of animal and human tissues via UPLC-MS. Nature Protocols, 8, 17–32.
Weiss, R. H., & Kim, K. (2011). Metabolomics in the study of kidney diseases. Nature Reviews Nephrology, 8, 22–33.
Wishart, D. S. (2016). Emerging applications of metabolomics in drug discovery and precision medicine. New England Journal of Medicine, 15, 473–484. https://doi.org/10.1038/nrd.2016.32.
Wouters, O. J., O’Donoghue, D. J., Ritchie, J., Kanavos, P. G., & Narva, A. S. (2015). Early chronic kidney disease: Diagnosis, management and models of care. Nature Reviews Nephrology, 11, 491–502. https://doi.org/10.1038/nrneph.2015.85.
Yu, B., Zheng, Y., Nettleton, J. A., Alexander, D., Coresh, J., & Boerwinkle, E. (2014). Serum metabolomic profiling and incident CKD among African Americans. Clinical Journal of the American Society of Nephrology, 9, 1410–1417. https://doi.org/10.2215/CJN.11971113.
Zhang, Z. H., Vaziri, N. D., Wei, F., Cheng, X. L., Bai, X., & Zhao, Y. Y. (2016a). An integrated lipidomics and metabolomics reveal nephroprotective effect and biochemical mechanism of Rheum officinale in chronic renal failure. Scientific Reports, 6, 22151. https://doi.org/10.1038/srep22151.
Zhang, Z. H., et al. (2016b). Metabolomic signatures of chronic kidney disease of diverse etiologies in the rats and humans. Journal of Proteome Research, 15, 3802–3812.
Zhao, Y. Y. (2013). Metabolomics in chronic kidney disease. Clinica Chimica Acta, 422, 59–69.
Zhao, Y. Y., et al. (2013). Intrarenal metabolomic investigation of chronic kidney disease and its TGF-beta1 mechanism in induced-adenine rats using UPLC Q-TOF/HSMS/MS(E). Journal of Proteome Research, 12, 2692–2703.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81872985, 81673578). No funding bodies had any role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YG and ZYY design and wrote the manuscript, DQC and YH performed data analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim that they do not have any conflict of interest.
Ethical approval
The part of patient study was approved by the Ethical Committee and all patients provided informed consent prior to entering the study. The present study has complied with all relevant ethical regulations. The sample collection was approved Shaanxi Traditional Chinese Medicine Hospital (Permit Number: SXSY-235610).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Y., Yu, H., Chen, D. et al. Machine learning distilled metabolite biomarkers for early stage renal injury. Metabolomics 16, 4 (2020). https://doi.org/10.1007/s11306-019-1624-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1624-0