Abstract
Introduction
Anabolic steroids are frequently misused for performance enhancement during sports competitions. One of the major bottlenecks in the confident analysis of steroids and their metabolites is the non-availability/cost of standard reference compounds.
Objective
The study objective was to identify the common metabolites of prohibited anabolic steroids that are produced in both fungi and human and thus can be synthesized in bulk using fungal cultures. Mesterolone is used as a case study.
Methods
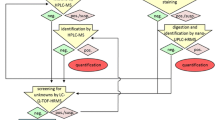
The study was conducted in three steps; we first studied the fungal transformation of mesterolone. In the second step, these metabolites were used as references to detect in human urine after the oral use of mesterolone using LC-ESI-QqQ-MS/MS. In the third step, 12 fungal cultures were screened to evaluate their potential to produce reference markers.
Results
This led to the detection of two metabolites, 6α-hydroxymesterolone (M1) and 7α-hydroxymesterolone (M2) that were found to be common in both, fungal cultures and human urine samples. Moreover, Rhizopus stolonifer and Beauveria bassiana can be considered as good candidates to produce M1 and M2 metabolites, respectively.
Conclusion
This approach can be employed for the synthesis of marker compounds of other prohibited anabolic steroids thus can be detected efficiently during national and international sports competitions.


Similar content being viewed by others
References
Abourashed, E. A., Clark, A. M., & Hufford, C. D. (1999). Microbial models of mammalian metabolism of xenobiotics: An updated review. Current Medicinal Chemistry, 6, 359–374.
Ahmad, M. S., et al. (2014). Biotransformation of androgenic steroid mesterolone with Cunninghamella blakesleeana and Macrophomina phaseolina. Steroids, 82, 53–59. doi:10.1016/j.steroids.2014.01.001.
Barwin, B. N., Clarke, S. D., Biggart, J. D., & Lamont, A. (1973). Mesterolone in the treatment of male infertility. Practitioner, 211, 669–674.
Brzezowska, E., Dmochowska-Gładysz, J., & Kołek, T. (1996). Biotransformation XXXIX. Metabolism of testosterone, androstenedione, progesterone and testosterone derivatives in Absidia coerulea culture. The Journal of Steroid Biochemistry and Molecular Biology, 57, 357–362. doi:10.1016/0960-0760(95)00279-0.
Choudhary, M. I., et al. (2005). Microbial transformation of mesterolone. Chemistry and Biodiversity, 2, 392–400. doi:10.1002/cbdv.200590019.
Corona, G., Rastrelli, G., Vignozzi, L., & Maggi, M. (2012). Emerging medication for the treatment of male hypogonadism. Expert Opinion on Emerging Drugs, 17, 239–259. doi:10.1517/14728214.2012.683411.
El Sayed, K. A. (2000). Microbial models of mammalian metabolism: microbial transformation of naproxen. Die Pharmazie, 55, 934–936.
Feneley, M. R., & Carruthers, M. (2012). Is testosterone treatment good for the prostate? Study of safety during long-term treatment. The journal of Sexual Medicine, 9, 2138–2149. doi:10.1111/j.1743-6109.2012.02808.x.
Fontana, K., White, K. E., Campos, G. E., da Cruz-Hofling, M. A., & Harris, J. B. (2010). Morphological changes in murine skeletal muscle in response to exercise and mesterolone. Journal of Electron Microscopy, 59, 153–164 doi:10.1093/jmicro/dfp053.
Georgakopoulos, C., Saugy, M., Giraud, S., Robinson, N., & Alsayrafi, M. (2012). Analytical progresses of the International Olympic Committee and World Anti-Doping Agency Olympic laboratories. Bioanalysis, 4, 1549–1563. doi:10.4155/bio.12.148.
Hartgens, F., & Kuipers, H. (2004). Effects of androgenic-anabolic steroids in athletes. Sports Medicine (Auckland, N. Z.), 34, 513–554.
Hezari, M., & Davis, P. J. (1992). Microbial models of mammalian metabolism. N-dealkylation of furosemide to yield the mammalian metabolite CSA using Cunninghamella elegans. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 20, 882–888.
Hezari, M., & Davis, P. J. (1993). Microbial models of mammalian metabolism. Furosemide glucoside formation using the fungus Cunninghamella elegans. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 21, 259–267.
Ho, E. N., et al. (2007). Metabolic studies of mesterolone in horses. Anal Chim Acta, 596, 149–155. doi:10.1016/j.aca.2007.05.052.
Huszcza, E., & Dmochowska-Gładysz, J. (2003). Transformations of testosterone and related steroids by Botrytis cinerea. Phytochemistry, 62, 155–158. doi:10.1016/S0031-9422(02)00490-9.
Kamber, M., & Mullis, P. E. (2010). The worldwide fight against doping: from the beginning to the World Anti-Doping Agency. Endocrinology and Metabolism Clinics of North America, 39, 1–9. doi:10.1016/j.ecl.2009.10.009.
Leone, G., & Petrosino, L. (1976). [Depot mesterolone in the management of male hypogonadism]. La Clinica terapeutica, 79, 255–261.
Lu, J., Fernandez-Alvarez, M., Yang, S., He, G., Xu, Y., & Aguilera, R. (2015). New potential biomarkers for mesterolone misuse in human urine by liquid chromatography quadrupole time-of-flight mass spectrometry. Journal of Mass Spectrometry and Ion Physics, 50, 153–159. doi:10.1002/jms.3508.
Mazzoni, I., Barroso, O., & Rabin, O. (2011). The list of prohibited substances and methods in sport: Structure and review process by the world anti-doping agency. Journal of Analytical Toxicology, 35, 608–612.
Moussa, C., Houziaux, P., Danree, B., & Azerad, R. (1997). Microbial models of mammalian metabolism. Fungal metabolism of phenolic and nonphenolic p-cymene-related drugs and prodrugs. II. Metabolites of nonphenolic derivatives. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 25, 311–316.
Musharraf, S. G., Ali, A., Khan, N. T., Yousuf, M., Choudhary, M. I., & Atta ur, R. (2013). Tandem mass spectrometry approach for the investigation of the steroidal metabolism: Structure–fragmentation relationship (SFR) in anabolic steroids and their metabolites by ESI-MS/MS analysis. Steroids, 78, 171–181. doi:10.1016/j.steroids.2012.10.017.
Pozo, O. J., et al. (2008). Collision-induced dissociation of 3-keto anabolic steroids and related compounds after electrospray ionization. Considerations for structural elucidation. Rapid Communications in Mass Spectrometry: RCM, 22, 4009–4024. doi:10.1002/rcm.3823.
Pozo, O. J., et al. (2009). Detection and characterization of a new metabolite of 17alpha-methyltestosterone. Drug Metabolism and Disposition: the Biological Fate of Chemicals, 37, 2153–2162. doi:10.1124/dmd.109.028373.
Rao, G. P., & Davis, P. J. (1997). Microbial models of mammalian metabolism. Biotransformations of HP 749 (besipirdine) using Cunninghamella elegans. Drug Metabolism and Disposition, 25, 709–715.
Schänzer, W., & Donike, M. (1993). Metabolism of anabolic steroids in man: synthesis and use of reference substances for identification of anabolic steroid metabolites. Analytica Chimica Acta, 275, 23–48. doi:10.1016/0003-2670(93)80274-O.
Schulz, H., & Niermann, H. (1974). [Depot mesterolone therapy in hypogonadism]. Fortschr Androl, 3, 191–197.
Sjoqvist, F., Garle, M., & Rane, A. (2008). Use of doping agents, particularly anabolic steroids, in sports and society. Lancet, 371, 1872–1882 doi:10.1016/S0140-6736(08)60801-6.
Songtao, B., lianxiang, D., Liming, Z., & Fuping, L. (2005). Bioconversion of methyl-testosterone in a biphasic system. Process Biochemistry, 40, 3309–3313. doi:10.1016/j.procbio.2005.03.019.
Yang, W., Jiang, T., Acosta, D., & Davis, P. J. (1993). Microbial models of mammalian metabolism: involvement of cytochrome P450 in the N-demethylation of N-methylcarbazole by Cunninghamella echinulata. Xenobiotica, 23, 973–982.
Acknowledgements
The authors are thankful to Mr. Muhammad Noman Khan, Mr. Faraz Ul Haq, Ms. Hamna Shadab and Ms. Adeeba Abbas for their assistance in UHPLC-MS/MS analysis and sample preparation. The authors are also thankful to the volunteers for taking part in this study. The authors would like to acknowledge the financial support by the Pakistan Academy of Sciences for the Project No. 5-9/EAS 54.
Author information
Authors and Affiliations
Contributions
SGM conceived the idea, supervised the study and wrote the manuscript. MIC, AR, SA conducted the fungal transformation, purification and characterization of metabolites. GS participated in fugal extract preparations. SGM, QA, AA, and FS contributed in the quantitative method development and sample analysis. All authors contributed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interests relevant to this study.
Ethical approval
The present study was approved by the Independent Ethic Committee of the principal investigating institute (ICCBS) (-027-HB-2017). All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Musharraf, S.G., Arfeen, Q.u., Ali, A. et al. Fungi as a source of marker compounds for the control of illicit use of drugs: mesterolone as a case study. Metabolomics 13, 150 (2017). https://doi.org/10.1007/s11306-017-1287-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1287-7