Abstract
Introduction/objectives
Incidence of esophageal adenocarcinoma (EA), an often fatal cancer, has increased sharply over recent decades. Several important risk factors (reflux, obesity, smoking) have been identified for EA and its precursor, Barrett’s esophagus (BE), but a key challenge remains in identifying individuals at highest risk, since most with reflux do not develop BE, and most with BE do not progress to cancer. Metabolomics represents an emerging approach for identifying novel biomarkers associated with cancer development.
Methods
We used targeted liquid chromatography-mass spectrometry (LC-MS) to profile 57 metabolites in 322 serum specimens derived from individuals with gastroesophageal reflux disease (GERD), BE, high-grade dysplasia (HGD), or EA, drawn from two well-annotated epidemiologic parent studies.
Results
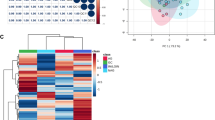
Multiple metabolites differed significantly (P < 0.05) between BE versus GERD (n = 9), and between HGD/EA versus BE (n = 4). Several top candidates (FDR q ≤ 0.15), including urate, homocysteine, and 3-nitrotyrosine, are linked to inflammatory processes, which may contribute to BE/EA pathogenesis. Multivariate modeling achieved moderate discrimination between HGD/EA and BE (AUC = 0.75), with less pronounced separation for BE versus GERD (AUC = 0.64).
Conclusion
Serum metabolite differences can be detected between individuals with GERD versus BE, and between those with BE versus HGD/EA, and may help differentiate patients at different stages of progression to EA.


Similar content being viewed by others
References
Abbassi-Ghadi, N., et al. (2013). Metabolomic profiling of oesophago-gastric cancer: A systematic review. European Journal of Cancer, 49, 3625–3637.
Allameh, A., et al. (2009). Immunohistochemical analysis of selected molecular markers in esophagus precancerous, adenocarcinoma and squamous cell carcinoma in Iranian subjects. Cancer Epidemiology, 33, 79–84.
Asiago, V. M., et al. (2010). Early detection of recurrent breast cancer using metabolite profiling. Cancer Research, 70, 8309–8318.
Baniasadi, H., et al. (2013). Targeted metabolic profiling of hepatocellular carcinoma and hepatitis C using LC-MS/MS. Electrophoresis, 34, 2910–2917.
Bobe, G., et al. (2010). Serum adiponectin, leptin, C-peptide, homocysteine, and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiology, Biomarkers and Prevention: a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 19, 1441–1452.
Carroll, P. A., et al. (2015). Deregulated myc requires mondoa/mlx for metabolic reprogramming and tumorigenesis. Cancer Cell, 27, 271–285.
Cook, M. B., et al. (2014). Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: A pooled analysis from the Barrett’s and Esophageal adenocarcinoma consortium (BEACON). PLoS One, 9, e103508.
Danese, S., et al. (2005). Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. The American journal of gastroenterology, 100, 886–895.
Dave, U., et al. (2004). In vitro 1 H-magnetic resonance spectroscopy of Barrett’s esophageal mucosa using magic angle spinning techniques. European Journal of Gastroenterology and Hepatology, 16, 1199–1205.
Davis, V. W., Schiller, D. E., Eurich, D., & Sawyer, M. B. (2012). Urinary metabolomic signature of esophageal cancer and Barrett’s esophagus. World Journal of Surgical Oncology, 10, 271.
Denkert, C., et al. (2006). Mass spectrometry-based metabolic profiling reveals different metabolite patterns in invasive ovarian carcinomas and ovarian borderline tumors. Cancer Research, 66, 10795–10804.
Djukovic, D., Baniasadi, H. R., Kc, R., Hammoud, Z., & Raftery, D. (2010). Targeted serum metabolite profiling of nucleosides in esophageal adenocarcinoma. Rapid Communications in Mass Spectrometry, 24, 3057–3062.
Doran, S. T., et al. (2003). Pathology of Barrett’s esophagus by proton magnetic resonance spectroscopy and a statistical classification strategy. American Journal of Surgery, 185, 232–238.
Edelstein, Z. R., Bronner, M. P., Rosen, S. N., & Vaughan, T. L. (2009). Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. The American Journal of Gastroenterology, 104, 834–842.
Edelstein, Z. R., Farrow, D. C., Bronner, M. P., Rosen, S. N., & Vaughan, T. L. (2007). Central adiposity and risk of Barrett’s esophagus. Gastroenterology, 133, 403–411.
Fanidi, A., et al. (2015). A prospective study of one-carbon metabolism biomarkers and cancer of the head and neck and esophagus. International Journal of Cancer. Journal International du Cancer, 136, 915–927.
Fini, M. A., Elias, A., Johnson, R. J., & Wright, R. M. (2012). Contribution of uric acid to cancer risk, recurrence, and mortality. Clin Transl Med, 1, 16.
Friedman, J., Hastie, T., & Tibshirani, R. (2010). Regularization paths for generalized linear models via coordinate descent. Journal of Statistical Software, 33, 1–22.
Galipeau, P. C., et al. (2007). NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Medicine, 4, 342–353.
Glickman, M. E., Rao, S. R., & Schultz, M. R. (2014). False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. Journal of Clinical Epidemiology, 67, 850–857.
Goldstein, S. R., Yang, G. Y., Chen, X., Curtis, S. K., & Yang, C. S. (1998). Studies of iron deposits, inducible nitric oxide synthase and nitrotyrosine in a rat model for esophageal adenocarcinoma. Carcinogenesis, 19, 1445–1449.
Gowda, G., et al. (2008). Metabolomics-based methods for early disease diagnostics. Expert Review of Molecular Diagnostics, 8, 617–633.
Gu, H., Gowda, G., & Raftery, D. (2012). Metabolic profiling: Are we en route to better diagnostic tests for cancer? Future Oncology, 8, 1207–1210.
Jiménez, P., et al. (2005). Free radicals and antioxidant systems in reflux esophagitis and Barrett’s esophagus. World journal of gastroenterology: WJG, 11, 2697–2703.
Kandulski, A., & Malfertheiner, P. (2011). Gastroesophageal reflux disease—from reflux episodes to mucosal inflammation. Nature Reviews Gastroenterology & Hepatology, 9, 15–22.
Kim, K., et al. (2014). Mealtime, temporal, and daily variability of the human urinary and plasma metabolomes in a tightly controlled environment. PLoS One, 9. doi:10.1371/journal.pone.0086223.
Kolonel, L. N., Yoshizawa, C., Nomura, A. M., & Stemmermann, G. N. (1994). Relationship of serum uric acid to cancer occurrence in a prospective male cohort. Cancer Epidemiology, Biomarkers and Prevention: a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 3, 225–228.
Li, X., et al. (2015). Assessment of esophageal adenocarcinoma risk using somatic chromosome alterations in longitudinal samples in Barrett’s esophagus. Cancer Prev Res (Phila), doi:10.1158/1940-6207.CAPR-15-0130.
Liesenfeld, D. B., Habermann, N., Owen, R. W., Scalbert, A., & Ulrich, C. M. (2013). Review of mass spectrometry-based metabolomics in cancer research. Cancer Epidemiology Biomarkers and Prevention, 22, 2182–2201.
Mayers, J. R., et al. (2014). Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature Medicine, 20, doi:10.1038/nm.3686.
Miller, J. W., et al. (2013). Homocysteine, cysteine, and risk of incident colorectal cancer in the Women’s Health Initiative observational cohort. The American Journal of Clinical Nutrition, 97, 827–834.
Nicholson, J. K., Lindon, J. C., & Holmes, E. (1999). Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica; The Fate of Foreign Compounds in Biological Systems, 29, 1181–1189.
O’Connell, T. M. (2012). Recent advances in metabolomics in oncology. Bioanalysis, 4, 431–451.
Phelan, J. J., et al. (2014). Differential expression of mitochondrial energy metabolism profiles across the metaplasia-dysplasia-adenocarcinoma disease sequence in Barrett’s oesophagus. Cancer Letters, 354, 122–131.
Reid, B. J., Blount, P. L., & Rabinovitch, P. S. (2003). Biomarkers in Barrett’s esophagus. Gastrointestinal Endoscopy Clinics of North America, 13, 369–397.
Reid, B. J., Li, X., Galipeau, P. C., & Vaughan, T. L. (2010). Barrett’s oesophagus and oesophageal adenocarcinoma: Time for a new synthesis. Nature Reviews Cancer, 10, 87–101.
Ross-Innes, C. S., et al. (2015). Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: A multi-center case-control study. PLoS Medicine, 12, e1001780.
Rubenstein, J. H., et al. (2013). Prediction of Barrett’s esophagus among men. The American Journal of Gastroenterology, 108, 353–362.
Sampliner, R. E. (2002). Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. American Journal of Gastroenterology, 97, 1888–1895.
Sampson, J. N., et al. (2013). Metabolomics in epidemiology: Sources of variability in metabolite measurements and implications. Cancer Epidemiology Biomarkers and Prevention, 22, 631–640.
Sanchez-Espiridion, B., et al. (2015). Identification of serum markers of esophageal adenocarcinoma by global and targeted metabolic profiling. Clinical Gastroenterology and Hepatology: the Official Clinical Practice Journal of the American Gastroenterological Association. doi:10.1016/j.cgh.2015.05.023.
Sing, T., Sander, O., Beerenwinkel, N., & Lengauer, T. (2005). ROCR: Visualizing classifier performance in R. Bioinformatics (Oxford, England), 21, 3940–3941.
Slupsky, C. M., et al. (2010). Urine metabolite analysis offers potential early diagnosis of ovarian and breast cancers. Clinical Cancer Research, 16, 5835–5841.
Sperber, H., et al. (2015). The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nature Cell Biology, 17, 1523–1535.
Spratlin, J. L., Serkova, N. J., & Eckhardt, S. G. (2009). Clinical applications of metabolomics in oncology: A review. Clinical Cancer Research, 15, 431–440.
Sreekumar, A., et al. (2009). Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature, 457, 910–914.
Strasak, A. M., et al. (2009). Use of penalized splines in extended Cox-type additive hazard regression to flexibly estimate the effect of time-varying serum uric acid on risk of cancer incidence: A prospective, population-based study in 78,850 men. Annals of Epidemiology, 19, 15–24.
Suchorolski, M. T., Paulson, T. G., Sanchez, C. A., Hockenbery, D., & Reid, B. J. (2013). Warburg and crabtree effects in premalignant Barrett’s esophagus cell lines with active mitochondria. PLoS One, 8, e56884.
Suzuki, M., Nishiumi, S., Matsubara, A., Azuma, T., & Yoshida, M. (2014). Metabolome analysis for discovering biomarkers of gastroenterological cancer. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 966, 59–69.
Thrift, A. P., Kendall, B. J., Pandeya, N., Vaughan, T. L., & Whiteman, D. C. (2012). A clinical risk prediction model for Barrett esophagus. Cancer Prevention Research, 5, 1115–1123.
Thrift, A. P., Kendall, B. J., Pandeya, N., & Whiteman, D. C. (2012). A model to determine absolute risk for Esophageal adenocarcinoma. Clinical Gastroenterology and Hepatology, 11, 138–44.e2.
Vaninetti, N. M., et al. (2008). Inducible nitric oxide synthase, nitrotyrosine and p53 mutations in the molecular pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Molecular Carcinogenesis, 47, 275–285.
Vaughan, T. L. (2014). From genomics to diagnostics of esophageal adenocarcinoma. Nature Genetics, 46, 806–807.
Vaughan, T. L., & Fitzgerald, R. C. (2015). Precision prevention of oesophageal adenocarcinoma. Nature Reviews Gastroenterology and Hepatology, 12, 243–248.
Weaver, J. M. J. et al. (2014). Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nature genetics, 46, 837–843.
Wikoff, W. R., et al. (2015). Diacetylspermine Is a novel prediagnostic serum biomarker for non-small-cell lung cancer and has additive performance with pro-surfactant protein B. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. doi:10.1200/JCO.2015.61.7779.
Xie, S.-H., & Lagergren, J. (2016). A model for predicting individuals’ absolute risk of esophageal adenocarcinoma: Moving towards tailored screening and prevention. International Journal of Cancer. Journal International du cancer. doi:10.1002/ijc.29988.
Yakoub, D., Keun, H. C., Goldin, R., & Hanna, G. B. (2010). Metabolic profiling detects field effects in nondysplastic tissue from esophageal cancer patients. Cancer Research, 70, 9129–9136.
Zhang, J., et al. (2011). Metabolomics study of esophageal adenocarcinoma. Journal of Thoracic and Cardiovascular Surgery, 141, 469–475.
Zhang, J., et al. (2012). Esophageal cancer metabolite biomarkers detected by LC-MS and NMR methods. PLoS One, 7, e30181.
Zhou, J., & Austin, R. C. (2009). Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. BioFactors (Oxford, England), 35, 120–129.
Zhu, J., et al. (2014). Colorectal cancer detection using targeted serum metabolic profiling. Journal of Proteome Research, 13, 4120–4130.
Acknowledgements
We thank Terri Watson, Tricia Christopherson, Paul Hansen, Jessica Arnaudo, David Cowan, and Carissa Sanchez for their contributions in project management, organization of biospecimens/data, or assistance with data retrieval. This work was principally supported by NIH grant R21CA178621 (T.L.V. and D.R.) and NIH training grant T32CA009168 (T.L.V). Additional support was provided by NIH grants K05CA124911 (T.L.V.), P01CA091955 (B.J.R), and 5P30CA015704 (D.R.).
Author contributions
Conception and design: M.F.B., T.L.V., D.R. Subject recruitment: T.L.V., B.J.R. Data acquisition: H.G., D.D., J.Z. Analysis and interpretation of data: M.F.B, H.G., D.D., L.O., D.R., T.L.V. Drafting of the manuscript: M.F.B., T.L.V., H.G., D.R. Study supervision: D.R., T.L.V. All authors critically revised the manuscript for intellectual content.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
D.R. holds equity and an executive role in Matrix‑Bio, Inc. (IN, USA). The authors have no other potential conflicts of interest to disclose.
Compliance with ethical requirements
All authors complied with ethical policies as described. Potential conflicts of interests were disclosed. This study included human participants and was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center. Appropriate informed consent was obtained.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buas, M.F., Gu, H., Djukovic, D. et al. Candidate serum metabolite biomarkers for differentiating gastroesophageal reflux disease, Barrett’s esophagus, and high-grade dysplasia/esophageal adenocarcinoma. Metabolomics 13, 23 (2017). https://doi.org/10.1007/s11306-016-1154-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1154-y