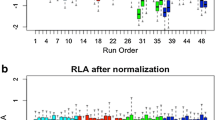
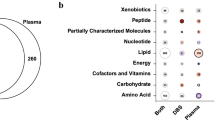
Abstract
Introduction
For pediatric diseases like childhood leukemia, a short latency period points to in-utero exposures as potentially important risk factors. Untargeted metabolomics of small molecules in archived newborn dried blood spots (DBS) offers an avenue for discovering early-life exposures that contribute to disease risks.
Objectives
The purpose of this study was to develop a quantitative method for untargeted analysis of archived newborn DBS for use in an epidemiological study (California Childhood Leukemia Study, CCLS).
Methods
Using experimental DBS from the blood of an adult volunteer, we optimized extraction of small molecules and integrated measurement of potassium as a proxy for blood hematocrit. We then applied this extraction method to 4.7-mm punches from 106 control DBS samples from the CCLS. Sample extracts were analyzed with liquid chromatography—high resolution mass spectrometry (LC-HRMS) and an untargeted workflow was used to screen for metabolites that discriminate population characteristics such as sex, ethnicity, and birth weight.
Results
Thousands of small molecules were measured in extracts of archived DBS. Normalizing for potassium levels removed variability related to varying hematocrit across DBS punches. Of the roughly 1000 prevalent small molecules that were tested, multivariate linear regression detected significant associations with ethnicity (three metabolites) and birth weight (15 metabolites) after adjusting for multiple testing.
Conclusions
This untargeted workflow can be used for analysis of small molecules in archived DBS to discover novel biomarkers, to provide insights into the initiation and progression of diseases, and to provide guidance for disease prevention.




Similar content being viewed by others
References
Abu-Rabie, P., Denniff, P., Spooner, N., Chowdhry, B. Z., & Pullen, F. S. (2015). Investigation of different approaches to incorporating internal standard in DBS quantitative bioanalytical workflows and their effect on nullifying hematocrit-based assay bias. Analytical Chemistry, 87(9), 4996–5003. doi:10.1021/acs.analchem.5b00908.
Anderson, M., & Braak, C. Ter (2003). Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation, 73(2), 85–113. doi:10.1080/00949650215733.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57(1), 289–300. doi:10.2307/2346101.
Bolstad, B. M., Irizarry, R., Astrand, M., & Speed, T. P. (2003). A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics (Oxford, England), 19(2), 185–193. doi:10.1093/bioinformatics/19.2.185.
Broeckling, C. D., Afsar, F. A., Neumann, S., Ben-Hur, A., & Prenni, J. E. (2014). RAMClust: a novel feature clustering method enables spectral-matching-based annotation for metabolomics data. Analytical Chemistry, 86(14), 6812–6817. doi:10.1021/ac501530d.
Bruce, S. J., Tavazzi, I., Parisod, V., Rezzi, S., Kochhar, S., & Guy, P. a (2009). Investigation of human blood plasma sample preparation for performing metabolomics using ultrahigh performance liquid chromatography/mass spectrometry. Analytical Chemistry, 81(9), 3285–3296. doi:10.1021/ac8024569.
California Department of Public Health (CDPH). (2016). Background and History of the California Biobank Program (CBP). https://www.cdph.ca.gov/programs/GDSP/Pages/MoreAboutTheCBP.aspx. Accessed 8 Aug 2016.
Capiau, S., Stove, V. V., Lambert, W. E., & Stove, C. P. (2013). Prediction of the hematocrit of dried blood spots via potassium measurement on a routine clinical chemistry analyzer. Analytical Chemistry, 85(1), 404–410. doi:10.1021/ac303014b.
Carlsen, S. M., Jacobsen, G., & Romundstad, P. (2006). Maternal testosterone levels during pregnancy are associated with offspring size at birth. European. Journal of Endocrinology: European Federation of Endocrine Societies, 155(2), 365–370. doi:10.1530/eje.1.02200.
Contrepois, K., Jiang, L., & Snyder, M. (2015). Optimized analytical procedures for the untargeted metabolomic profiling of human urine and plasma by combining hydrophilic interaction (HILIC) and reverse-phase liquid chromatography (RPLC)–mass spectrometry. Molecular & Cellular Proteomics, 14(6), 1684–1695. doi:10.1074/mcp.M114.046508.
De Kesel, P. M. M., Capiau, S., Stove, V. V., Lambert, W. E., & Stove, C. P. (2014). Potassium-based algorithm allows correction for the hematocrit bias in quantitative analysis of caffeine and its major metabolite in dried blood spots. Analytical and Bioanalytical Chemistry, 406(26), 6749–6755. doi:10.1007/s00216-014-8114-z.
den Burger, J.C.G., Wilhelm, A. J., Chahbouni, A. C., Vos, R. M., Sinjewel, A., & Swart, E. L. (2015). Haematocrit corrected analysis of creatinine in dried blood spots through potassium measurement. Analytical and Bioanalytical Chemistry, 407(2), 621–627.
Dénes, J., Szabó, E., Robinette, S. L., Szatmári, I., Szőnyi, L., Kreuder, J. G., et al. (2012). Metabonomics of newborn screening dried blood spot samples: A novel approach in the screening and diagnostics of inborn errors of metabolism. Analytical Chemistry, 84(22), 10113–10120. doi:10.1021/ac302527m.
Edmands, W. M. (2016). CompMS2miner: a metabolite identification R package. https://github.com/WMBEdmands/CompMS2miner. doi:10.5281/zenodo.56582. Accessed 29 September 2016.
Edmands, W. M., Barupal, D. K., & Scalbert, A. (2015). MetMSLine: An automated and fully integrated pipeline for rapid processing of high-resolution LC-MS metabolomic datasets. Bioinformatics (Oxford, England), 31(5), 788–790. doi:10.1093/bioinformatics/btu705.
Funk, W. E., McGee, J. K., Olshan, A. F., & Ghio, A. J. (2013). Quantification of arsenic, lead, mercury and cadmium in newborn dried blood spots. Biomarkers: Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals, 18(2), 174–177. doi:10.3109/1354750X.2012.750379.
Gonzales, J. L. (2011). Ethics for the pediatrician: Genetic testing and newborn screening. Pediatrics in Review, 32(11), 490–493. doi:10.1542/pir.32-11-490.
Hsu, F. F., & Turk, J. (2000). Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: A mechanistic study. Journal of the American Society for Mass Spectrometry, 11(11), 986–999. doi:10.1016/S1044-0305(00)00172-0.
Kim, B., Lee, M. N., Park, H. D., Kim, J. W., Chang, Y. S., Park, W. S., & Lee, S. Y. (2015). Dried blood spot testing for seven steroids using liquid chromatography-tandem mass spectrometry with reference interval determination in the Korean population. Annals of Laboratory Medicine, 35(6), 578–585. doi:10.3343/alm.2015.35.6.578.
Koeth, R. A., Wang, Z., Levison, B. S., Buffa, J. A., Org, E., Sheehy, B. T., et al. (2013). Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine, 19, 576–585. doi:10.1038/nm.3145.
Koulman, A., Prentice, P., Wong, M. C. Y., Matthews, L., Bond, N. J., Eiden, M., et al. (2014). The development and validation of a fast and robust dried blood spot based lipid profiling method to study infant metabolism. Metabolomics, 1–8. doi:10.1007/s11306-014-0628-z.
Lagiou, P., Samoli, E., Hsieh, C. C., Lagiou, A., Xu, B., Yu, G. P., et al. (2014). Maternal and cord blood hormones in relation to birth size. European Journal of Epidemiology, 29(5), 343–351. doi:10.1007/s10654-014-9914-3.
Liu, G., Mühlhäusler, B. S., & Gibson, R. A. (2014). A method for long term stabilisation of long chain polyunsaturated fatty acids in dried blood spots and its clinical application. Prostaglandins Leukotrienes and Essential Fatty Acids, 91(6), 251–260. doi:10.1016/j.plefa.2014.09.009.
Ma, W.-L., Gao, C., Bell, E. M., Druschel, C. M., Caggana, M., Aldous, K. M., et al. (2014). Analysis of polychlorinated biphenyls and organochlorine pesticides in archived dried blood spots and its application to track temporal trends of environmental chemicals in newborns. Environmental Research, 133, 204–210. doi:10.1016/j.envres.2014.05.029.
Metayer, C., Zhang, L., Wiemels, J. L., Bartley, K., Schiffman, J., Ma, X., et al. (2013). Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiology, Biomarkers, & Prevention, 22(9), 1600–1611. doi:10.1158/1055-9965.EPI-13-0350.
Michopoulos, F., Theodoridis, G., Smith, C. J., & Wilson, I. D. (2010). Metabolite profiles from dried biofluid spots for metabonomic studies using UPLC combined with oaToF-MS. Journal of Proteome Research, 9(6), 3328–3334. doi:10.1021/pr100124b.
Mitchell, F., & Shackleton, C. H. (1969). The investigation of steroid metabolism in early infancy. In O. Bodansky & C. P. Stewart (Eds.), Advances in clinical chemsitry (Vol. 12, pp. 141–215). New York: Academic Press.
Nakagawa, S., & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution, 4(2), 133–142. doi:10.1111/j.2041-210x.2012.00261.x.
Niinivirta, K., Isolauri, E., Laakso, P., Linderborg, K., & Laitinen, K. (2011). Dietary counseling to improve fat quality during pregnancy alters maternal fat intake and infant essential fatty acid status. The Journal of Nutrition, 141(7), 1281–1285. doi:10.3945/jn.110.137083.
Nochera, C., Goossen, L., Brutus, A., Cristales, M., & Eastman, B. (2011). Consumption of DHA + ePA by low-income women during pregnancy and lactation. Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition, 26(4), 445–450.
Patti, G. J., Tautenhahn, R., & Siuzdak, G. (2012). Meta-analysis of untargeted metabolomic data from multiple profiling experiments. Nature Protocols, 7(3), 508–516. doi:10.1038/nprot.2011.454.
Prentice, P., Koulman, A., Matthews, L., Acerini, C. L., Ong, K. K., & Dunger, D. B. (2015). Lipidomic analyses, breast- and formula-feeding, and growth in infants. Journal of Pediatrics, 166(2), 276–281.e6. doi:10.1016/j.jpeds.2014.10.021.
Pupillo, D., Simonato, M., Cogo, P. E., Lapillonne, A., & Carnielli, V. P. (2016). Short-term stability of whole blood polyunsaturated fatty acid content on filter paper during storage at −28 °C. Lipids, 51(2), 193–198. doi:10.1007/s11745-015-4111-z.
R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/.
Rajesh, G. D., Bhat, B. V., Sridhar, M. G., & Ranganathan, P. (2000). Growth hormone levels in relation to birth weight and gestational age. Indian Journal of Pediatrics, 67(3), 175–177. doi:10.1007/BF02723656.
Raju, K. S. R., Taneja, I., Rashid, M., Sonkar, A. K., Wahajuddin, M., & Singh, S. P. (2016). DBS-platform for biomonitoring and toxicokinetics of toxicants: Proof of concept using LC-MS/MS analysis of fipronil and its metabolites in blood. Scientific Reports, 6, 22447. doi:10.1038/srep22447.
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., & Smyth, G. K. (2015). Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research, 43(7), e47. doi:10.1093/nar/gkv007.
Sánchez-Guijo, A., Oji, V., Hartmann, M. F., Traupe, H., & Wudy, S. A. (2015). Simultaneous quantification of cholesterol sulfate, androgen sulfates, and progestagen sulfates in human serum by LC-MS/MS. Journal of Lipid Research, 56(9), 1843–1851. doi:10.1194/jlr.D061499.
Schindler, A., & Siiteri, P. (1968). Isolation and quantitation of steroids from normal human amniotic fluid. Journal of Clinical Endocrinology and Metabolism Endocrinology, 28, 1189098. doi:10.1210/jcem-28-8-1189.
Schwarz, E., Liu, A., Randall, H., Haslip, C., Keune, F., Murray, M., et al. (2009). Use of steroid profiling by UPLC-MS/MS as a second tier test in newborn screening for congenital adrenal hyperplasia: The Utah experience. Pediatric Research, 66(2), 230–235. doi:10.1203/PDR.0b013e3181aa3777.
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., Amin, N., Schwikowski, B., & Ideker, T. (2003). Cytoscoape: A software environment for integrated models of biomolecular interaction networks. Genome Research, 12, 2498–2504. doi:10.1101/gr.1239303.
Smith, C., Elizabeth, J., O’Maille, G., Abagyan, R., & Siuzdak, G. (2006). XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Analytical Chemistry, 78(3), 779–787. doi:10.1021/ac051437y.
Smith, C. A., O’maille, G., Want, E. J., Qin, C., Trauger, S. A., Brandon, T. R., et al. (2005). METLIN A metabolite mass spectral database. Proceedings of the 9th International Congress of Therapeutic Drug Monitoring & Clinical Toxicology, 27(6), 747–751. doi:10.1097/01.ftd.0000179845.53213.39.
Tang, W. H. W., Wang, Z. E., Levison, B. S., Koeth, R. A., Britt, E. B., Fu, X. M., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine, 368(17), 1575–1584. doi:10.1056/NEJMoa1109400.
Vu, D. H., Koster, R. A., Alffenaar, J. W. C., Brouwers, J. R. B. J., & Uges, D. R. A. (2011). Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. Journal of Chromatography B, 879(15–16), 1063–1070. doi:10.1016/j.jchromb.2011.03.017.
Wang, Z., Klipfell, E., Bennett, B. J., Koeth, R., Levison, B. S., Dugar, B., et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 472(7341), 57–63. doi:10.1038/nature09922.
Wiemels, J., Cazzaniga, G., Daniotti, M., Eden, O., Addison, G., Masera, G., et al. (1999). Prenatal origin of acute lymphoblastic leukaemia in children. Lancet, 354(9189), 1499–1503. doi:10.1016/S0140-6736(99)09403-9.
Wishart, D. S., Jewison, T., Guo, A. C., Wilson, M., Knox, C., Liu, Y., et al. (2013). HMDB 3.0-The Human Metabolome Database in 2013. Nucleic Acids Research, 41(D1), 801–807. doi:10.1093/nar/gks1065.
Youhnovski, N., Bergeron, A., Furtado, M., & Garofolo, F. (2011). Pre-cut dried blood spot (PCDBS): an alternative to dried blood spot (DBS) technique to overcome hematocrit impact. Rapid Communications in Mass Spectrometry: RCM, 25(19), 2951–2958. doi:10.1002/rcm.5182.
Acknowledgements
We gratefully acknowledge the assistance of Agilent Technologies (Santa Clara, CA, USA) for the loans of the liquid-chromatography mass-spectrometry instruments used in these analyses. We also thank the families for their participation in the CCLS.
Funding
This work was supported by the National Institute for Environmental Health Sciences of the U.S. National Institutes of Health (NIEHS) and the U.S. Environmental Protection Agency through grants to the Center for Integrative Research on Childhood Leukemia and the Environment (NIEHS grants P01 ES018172 and P50ES018172 and USEPA grants RD83451101 and RD83615901), by the California Childhood Leukemia Study (NIEHS grants R01ES009137 and P42ES004705), by NIEHS grant P42ES0470518, and by a post-doctoral fellowship from the Environment and Health Fund, Jerusalem, Israel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. Petrick, W. Edmands, C. Schiffman, H. Grigoryan, K. Perttula, Y. Yano, S. Dudoit, T. Whitehead, C. Metayer and S. Rappaport have no conflict of interest to declare.
Disclaimer
The ideas and opinions expressed herein are those of the authors and do not necessarily represent the official views of EPA or NIEHS. Endorsement of any product or service mentioned is not intended nor should it be inferred.
Ethics approval
The study was approved by the University of California Committee for the Protection of Human Subjects, the California Health and Human Services Agency Committee for the Protection of Human Subjects, and the institutional review boards of all participating hospitals.
Informed consent
Written informed consent was obtained from the adult volunteer subject and parents of all participating subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petrick, L., Edmands, W., Schiffman, C. et al. An untargeted metabolomics method for archived newborn dried blood spots in epidemiologic studies. Metabolomics 13, 27 (2017). https://doi.org/10.1007/s11306-016-1153-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1153-z