Abstract
Introduction
Meningitis, a morbidly infectious central nervous system pathology is accompanied by acute inflammation of the meninges, causing raised intracranial pressure linked with serious neurological sequelae.
Objective
To observe the variation in the metabolic profile, that may occur in serum and urine along with CSF in adults using 1H NMR spectroscopy, with an attempt of appropriate and timely treatment regimen.
Methods
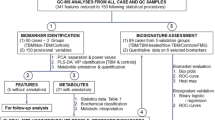
The 1H NMR-based metabolomics has been performed in 115 adult subjects for differentiating bacterial meningitis (BM) and tubercular meningitis (TBM).
Results
The discriminant function analysis (DFA) of the three bio-fluids collectively identified 3-hydroxyisovalerate, lactate, glucose, formate, valine, alanine, ketonic bodies, malonate and choline containing compounds (choline and GPC) as significant metabolites among cases versus control group. The differentiation of bacterial meningitis and tuberculous meningitis (BM vs. TBM) can be done on the basis of identification of 3-hydroxyisovalerate, isobutyrate and formate in case of CSF (with a correct classification of 78 %), alanine in serum (correct classification 60 %), valine and acetone in case of urine (correct classification 89.1 %). The NMR spectral bins based orthogonal signal correction principal component analysis score plots of significant metabolites obtained from DFA also provided group classification among cases versus control group in CSF, serum and urine samples. The variable importance in projection scores also identified similar significant metabolites as obtained from DFA, collectively in CSF, serum and urine samples, responsible for differentiation of meningitis.
Conclusion
The CSF contained metabolites which are formed during infection and inflammation, and these were also found in significant quantity in serum and urine samples.




Similar content being viewed by others
Abbreviations
- BM:
-
Bacterial meningitis/pyogenic meningitis
- CPMG:
-
Carr-purcell-meiboom-gill
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- DFA:
-
Discriminant function analysis
- FDR:
-
False discovery rate
- MRI:
-
Magnetic resonance imaging
- NMR:
-
Nuclear magnetic resonance
- NOESY:
-
Nuclear overhauser effect spectroscopy
- OSC-PCA:
-
Orthogonal signal correction-principal component analysis
- PLS-DA:
-
Partial least square discriminant analysis
- SD:
-
Standard deviation
- TBM:
-
Tubercular meningitis
- TSP:
-
Trimethylsilyl propionate
- VIP:
-
Variables importance in projection
References
Ahuja, G. K., Mohan, K. K., Prasad, K., & Behari, M. (1994). Diagnostic criteria for tuberculous meningitis and their validation. Tubercle and Lung Disease, 75(2), 149–152.
Beckonert, O., Keun, H. C., Ebbels, T. M., Bundy, J., Holmes, E., Lindon, J. C., et al. (2007). Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nature Protocols, 2(11), 2692–2703.
Bharti, S. K., & Roy, R. (2012). Quantitative 1H NMR spectroscopy. Trends in Analytical Chemistry, 35, 5–26.
Botha, H., Ackerman, C., Candy, S., Carr, J. A., Griffith-Richards, S., & Bateman, K. J. (2012). Reliability and diagnostic performance of CT imaging criteria in the diagnosis of tuberculous meningitis. PLoS One, 7(6), e38982. doi:10.1371/journal.pone.0038982.
Coen, M., O’Sullivan, M., Bubb, W. A., Kuchel, P. W., & Sorrell, T. (2005). Proton nuclear magnetic resonance-based metabonomics for rapid diagnosis of meningitis and ventriculitis. Clinical Infectious Disease, 41(11), 1582–1590.
Cunha, B. A. (2004). The diagnostic usefulness of cerebrospinal fluid lactic acid levels in central nervous system infections. Clinical Infectious Disease, 39(8), 1260–1261.
Das, B. K., Gurubacharya, R. L., Mohapatra, T. M., & Mishra, O. P. (2003). Bacterial antigen detection test in meningitis. Indian Journal of Pediatrics, 70(10), 799–801.
Emwas, A. H., Luchinat, C., Turano, P., Tenori, L., Roy, R., Salek, R. M., et al. (2015). Standardizing the experimental conditions for using urine in NMR-based metabolomic studies with a particular focus on diagnostic studies: a review. Metabolomics, 11(4), 872–894.
Eoh, H., & Rhee, K. Y. (2013). Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proceedings of the National Academy of the Sciences of the United States of America, 110(16), 6554–6559. doi:10.1073/pnas.1219375110.
Farrant, R. D., Hollerton, J. C., Lynn, S. M., Provera, S., Sidebottom, P. J., & Upton, R. J. (2010). NMR quantification using an artificial signal. Magnetic Resonance in Chemistry, 48(10), 753–762. doi:10.1002/mrc.2647.
Fisher, R. A., & Yates, F. (Eds.). (1957). Statistical tables for biological, agricultural, and medical research (5th ed.). Edinburgh: Oliver and Boyd.
Gupta, R. K., Vatsal, D. K., Husain, N., Chawla, S., Prasad, K. N., Roy, R., et al. (2001). Differentiation of tuberculous from pyogenic brain abscesses with in vivo proton MR spectroscopy and magnetization transfer MR imaging. American Journal of Neuroradiology, 22(8), 1503–1509.
Hasbun, R., Bijlsma, M., Brouwer, M. C., Khoury, N., Hadi, C. M., van der, E. A., et al. (2013). Risk score for identifying adults with CSF pleocytosis and negative CSF Gram stain at low risk for an urgent treatable cause. The Journal of Infection, 67(2), 102–110. doi:10.1016/j.jinf.2013.04.002.
Huy, N. T., Thao, N. T., Diep, D. T., Kikuchi, M., Zamora, J., & Hirayama, K. (2010). Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Critical Care, 14(6), 1–15. doi:10.1186/cc9395.
Kabra, S. K., Kumar, P., Verma, I. C., Mukherjee, D., Chowdhary, B. H., Sengupta, S., et al. (1991). Bacterial meningitis in India: an IJP survey. Indian Journal of Pediatrics, 58(4), 505–511.
Khovidhunkit, W., Kim, M. S., Memon, R. A., Shigenaga, J. K., Moser, A. H., Feingold, K. R., et al. (2004). Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. Journal of Lipid Research, 45(7), 1169–1196.
Lannigan, R., MacDonald, M. A., Marrie, T. J., & Haldane, E. V. (1980). Evaluation of cerebrospinal fluid lactic acid levels as an aid in differential diagnosis of bacterial and viral meningitis in adults. Journal of Clinical Microbiology, 11(4), 324–327.
Martin, F. P., Sprenger, N., Yap, I. K., Wang, Y., Bibiloni, R., Rochat, F., et al. (2009). Panorganismal gut microbiome-host metabolic crosstalk. Journal of Proteome Research, 8(4), 2090–2105. doi:10.1021/pr801068x.
Mason, S., van Furth, A. M., Mienie, L. J., Engelke, U. F., Wevers, R. A., Solomons, R., et al. (2015). A hypothetical astrocyte-microglia lactate shuttle derived from a 1H NMR metabolomics analysis of cerebrospinal fluid from a cohort of South African children with tuberculous meningitis. Metabolomics, 11(4), 822–837.
McNally, M. A., & Hartman, A. L. (2012). Ketone bodies in epilepsy. Journal of Neurochemistry, 121(1), 28–35. doi:10.1111/j.1471-4159.2012.07670.x.
Mehta, A., Mahale, R. R., Sudhir, U., Javali, M., & Srinivasa, R. (2015). Utility of cerebrospinal fluid cortisol level in acute bacterial meningitis. Annals of Indian Academy of Neurology, 18(2), 210–214. doi:10.4103/0972-2327.150626.
Michinaga, S., & Koyama, Y. (2015). Pathogenesis of brain edema and investigation into anti-edema drugs. International Journal of Molecular Sciences, 16(5), 9949–9975. doi:10.3390/ijms16059949.
Murthy, J. M. (2005). Management of intracranial pressure in tuberculous meningitis. Neurocritical Care, 2(3), 306–312.
Naghavi, M., Wang, H., Lozano, R., Davis, A., Liang, X., Zhou, M., et al. (2015). Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, 385(9963), 117–171. doi:10.1016/S0140-6736(14)61682-2.
Nau, R., & Brück, W. (2002). Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends in Neurosciences, 25(1), 38–45.
Nigrovic, L. E., Kimia, A. A., Shah, S. S., & Neuman, M. I. (2012). Relationship between cerebrospinal fluid glucose and serum glucose. The New England Journal of Medicine, 366(6), 576–578. doi:10.1056/NEJMc1111080.
Niu, Y. C., Feng, R. N., Hou, Y., Li, K., Kang, Z., Wang, J., et al. (2012). Histidine and arginine are associated with inflammation and oxidative stress in obese women. The British Journal of Nutrition, 108(1), 57–61. doi:10.1017/S0007114511005289.
Pearce, J. M. (1994). Walter Eassex Wynter, Quincke, and lumbar puncture. Journal of Neurology, Neurosurgery and Psychiatry, 57(2), 179.
Philip, N., William, T., & William, D. V. (2015). Diagnosis of tuberculous meningitis: challenges and promises. The Malaysian Journal of Pathology, 37(1), 1–9.
Prasad, K., & Sahu, J. K. (2011). Cerebrospinal fluid lactate: is it a reliable and valid marker to distinguish between acute bacterial meningitis and aseptic meningitis? Critical Care, 15(1), 104. doi:10.1186/cc9396.
Ross, B. D. (1991). Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR in Biomedicine, 4(2), 59–63.
Sáez-Llorens, X., & McCracken, G. H. (2003). Bacterial meningitis in children. The Lancet, 361(9375), 2139–2148.
Sakushima, K., Hayashino, Y., Kawaguchi, T., Jackson, J. L., & Fukuhara, S. (2011). Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. The Journal of Infection, 62(4), 255–262. doi:10.1016/j.jinf.2011.02.010.
Scremin, O. U., & Jenden, D. J. (1989). Focal ischemia enhances choline output and decreases acetylcholine output from rat cerebral cortex. Stroke, 20(1), 92–95.
Solari, L., Soto, A., Agapito, J. C., Acurio, V., Vargas, D., Battaglioli, T., et al. (2013). The validity of cerebrospinal fluid parameters for the diagnosis of tuberculous meningitis. International Journal of Infectious Diseases, 17(12), e1111–e1115. doi:10.1016/j.ijid.2013.06.003.
Spanos, A., Harrell, F. E., & Durack, D. T. (1989). Differential diagnosis of acute meningitis. An analysis of the predictive value of initial observations. JAMA, 262(19), 2700–2707.
Stenina, M. A., Voevodin, D. A., Stakhanov, V. D., Kisilevich, O. N., & Rozanova, G. N. (2003). Tissue hypoxia and intestinal dysbiosis in children with tuberculosis. Bulletin of Experimental Biology and Medicine, 135(2), 178–180.
Subramanian, A., Gupta, A., Saxena, S., Gupta, A., Kumar, R., Nigam, A., et al. (2005). Proton MR CSF analysis and a new software as predictors for the differentiation of meningitis in children. NMR in Biomedicine, 18(4), 213–225.
Sweatt, A. J., Wood, M., Suryawan, A., Wallin, R., Willingham, M. C., & Hutson, S. M. (2004). Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. American Journal of Physiology, Endocrinology and Metabolism, 286(1), E64–E76.
Tachikawa, M., Fujinawa, J., Takahashi, M., Kasai, Y., Fukaya, M., Sakai, K., et al. (2008). Expression and possible role of creatine transporter in the brain and at the blood-cerebrospinal fluid barrier as a transporting protein of guanidinoacetate, an endogenous convulsant. Journal of Neurochemistry, 107(3), 768–780. doi:10.1111/j.1471-4159.2008.05652.x.
Tai, M. S. (2013). Tuberculous meningitis: diagnostic and radiological features, Pathogenesis and Biomarkers. Neuroscience and Medicine, 4(2), 101–107. doi:10.4236/nm.2013.42016.
Taylor, J. (1845). On some of the causes of pericarditis, especially acute rheumatism, and Bright’s disease of the kidneys, with incidental observations on the frequency, and on some of the causes of various other internal inflammations. Medico-Chirurgical Transactions, 28, 453–570.
Thwaites, G. E., van Toorn, R., & Schoeman, J. (2013). Tuberculous meningitis: more questions, still too few answers. The Lancet Neurology, 12(10), 999–1010. doi:10.1016/S1474-4422(13)70168-6.
Van Walsum, A. M. V. C., Jongsma, H. W., Wevers, R. A., Nijhuis, J. G., Crevels, J., Engelke, U. F., et al. (2002). 1H-NMR spectroscopy of cerebrospinal fluid of fetal sheep during hypoxia-induced acidemia and recovery. Pediatric Research, 52(1), 56–63.
Walter, A., Korth, U., Hilgert, M., Hartmann, J., Weichel, O., Hilgert, M., et al. (2004). Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiology of Ageing, 25(10), 1299–1303.
Wannemacher, R. W., Pace, J. G., Beall, R. A., Dinterman, R. E., Petrella, V. J., & Neufeld, H. A. (1979). Role of the liver in regulation of ketone body production during sepsis. The Journal of Clinical Investigation, 64(6), 1565–1572.
Yesilkaya, H., Spissu, F., Carvalho, S. M., Terra, V. S., Homer, K. A., Benisty, R., et al. (2009). Pyruvate formate lyase is required for pneumococcal fermentative metabolism and virulence. Infection and Immunity, 77(12), 5418–5427. doi:10.1128/IAI.00178-09.
Youssef, F. G., Afifi, S. A., Azab, A. M., Wasfy, M. M., Abdel-Aziz, K. M., Parker, T. M., et al. (2006). Differentiation of tuberculous meningitis from acute bacterial meningitis using simple clinical and laboratory parameters. Diagnostic Microbiology and Infectious Disease, 55(4), 275–278.
Acknowledgments
The authors are thankful to Dr. Ram Manohar Lohia Institute of Medical Sciences for intramural funding (IEC 14/11) to carry out the study and Centre of Biomedical Research, Lucknow where the 1H NMR spectroscopy was conducted. Dr. Suruchi Singh would like to extend her thanks to Council of Scientific and Industrial Research (RA Award No.: 09/916/(0077)/2013/EMR-I) for financial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no potential conflict of interest. The disclosure of potential conflict of interest in the prescribed format has been obtained from all the authors.
Ethical approval
The study was ethically approved and the work was performed in strict accordance with the guidelines of Institutional Ethical Committee of Dr. Ram Manohar Lohia Institute of Medical Sciences (IEC 14/11).
Informed consent
The subjects were explained the study procedure and written and informed consent were obtained from them prior to the study. The authors: Tanushri Chatterji, Suruchi Singh, Manodeep Sen, Ajai Kumar Singh, Pradeep Kumar Maurya, Nuzhat Husain, Janmejai Kumar Srivastava, Sudhir Kumar Mandal, and Raja Roy are aware of ethical policy.
Additional information
Tanushri Chatterji and Suruchi Singh have contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chatterji, T., Singh, S., Sen, M. et al. Comprehensive 1H NMR metabolic profiling of body fluids for differentiation of meningitis in adults. Metabolomics 12, 130 (2016). https://doi.org/10.1007/s11306-016-1073-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-016-1073-y