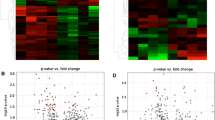
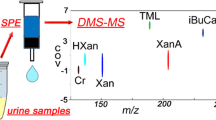
Abstract
The potential for exposures to ionizing radiation (IR) has increased in recent years. Although advances have been made, understanding the global metabolic response as a function of both dose and exposure time is challenging considering the complexity of the responses. Herein we report our findings on the dose- and time-dependency of the urinary response to IR in the male rat using radiation metabolomics. Urine samples were collected from adult male rats, exposed to 0.5–10 Gy γ-radiation, both before from 6 to 72 h following exposures. Samples were analyzed by liquid chromatography coupled with time-of-flight mass spectrometry, and deconvoluted mass chromatographic data were initially analyzed by principal component analysis. However, the breadth and complexity of the data necessitated the development of a novel approach to summarizing biofluid constituents after exposure, called Visual Analysis of Metabolomics Package (VAMP). VAMP revealed clear urine metabolite profile differences to as little as 0.5 Gy after 6 h exposure. Via VAMP, it was discovered that the response to radiation exposure found in rat urine is characterized by an overall net down-regulation of ion excretion with only a modest number of ions excreted in excess over pre-exposure levels. Our results show both similarities and differences with the published mouse urine response and a dose- and time-dependent net decrease in urine ion excretion associated with radiation exposure. These findings mark an important step in the development of minimally invasive radiation biodosimetry. VAMP should have general applicability in metabolomics to visualize overall differences and trends in many sample sets.





Similar content being viewed by others
Abbreviations
- DMS–MS:
-
Differential mobility spectrometry–mass spectrometry
- AFRRI:
-
Armed Forces Radiobiology Research Institute
- MS:
-
Mass spectrometer
- UPLC–TOFMS:
-
Ultra-performance liquid chromatography–time of flight mass spectrometry
- ESI:
-
Electrospray ionization
- ESI+:
-
Positive ESI
- ESI−:
-
Negative ESI
- PCA:
-
Principal component analysis
- PC:
-
Principal component
- IR:
-
Ionizing radiation
- IS:
-
Internal standard
- ppm:
-
Parts per million
- CI:
-
Confidence interval
- VAMP:
-
Visual Analysis of Metabolomics Package
References
Amendola, R., Basso, E., Pacifici, P. G., Piras, E., Giovanetti, A., Volpato, C., et al. (2006). Ret, Abl1 (cAbl) and Trp53 gene fragmentations in comet-FISH assay act as in vivo biomarkers of radiation exposure in C57BL/6 and CBA/J mice. Radiation Research, 165, 553–561.
Castro-Perez, J., Plumb, R., Granger, J. H., Beattie, I., Joncour, K., & Wright, A. (2005). Increasing throughput and information content for in vitro drug metabolism experiments using ultra-performance liquid chromatography coupled to a quadrupole time-of-flight mass spectrometer. Rapid Communications in Mass Spectrometry, 19, 843–848.
Chen, C., Brenner, D. J., & Brown, T. R. (2011). Identification of urinary biomarkers from X-irradiated mice using NMR spectroscopy. Radiation Research, 175, 622–630.
Christodouleas, J. P., Forrest, R. D., Ainsley, C. G., Tochner, Z., Hahn, S. M., & Glatstein, E. (2011). Short-term and long-term health risks of nuclear-power-plant accidents. New England Journal of Medicine, 364, 2334–2341.
Coy, S. L., Cheema, A. K., Tyburski, J. B., Laiakis, E. C., Collins, S. P., & Fornace, A. J. (2011). Radiation metabolomics and its potential in biodosimetry. International Journal of Radiation Biology, 87, 802–823.
Coy, S. L., Krylov, E. V., Schneider, B. B., Covey, T. R., Brenner, D. J., Tyburski, J. B., et al. (2010). Detection of radiation-exposure biomarkers by differential mobility prefiltered mass spectrometry (DMS-MS). International Journal of Mass Spectrometry, 291, 108–117.
Dewey, W. C., & Humphrey, R. M. (1965). Increase in radiosensitivity to ionizing radiation related to replacement of thymidine in mammalian cells with 5-bromodeoxyuridine. Radiation Research, 26, 538–553.
Dizdaroglu, M., & Simic, M. G. (1984). Radiation-induced crosslinking of cytosine. Radiation Research, 100, 41–46.
Dunn, S. R., Qi, Z., Bottinger, E. P., Breyer, M. D., & Sharma, K. (2004). Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney International, 65, 1959–1967.
Grace, M. B., Moyer, B. R., Prasher, J., Cliffer, K. D., Ramakrishnan, N., Kaminski, J., et al. (2010). Rapid radiation dose assessment for radiological public health emergencies: Roles of NIAID and BARDA. Health Physics, 98, 172–178.
Hafer, N., Cassatt, D., Dicarlo, A., Ramakrishnan, N., Kaminski, J., Norman, M. K., et al. (2010). NIAID/NIH radiation/nuclear medical countermeasures product research and development program. Health Physics, 98, 903–905.
Johnson, C. H., Patterson, A. D., Krausz, K. W., Kalinich, J. F., Tyburski, J. B., Kang, D. W., et al. (2012). Radiation Metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiation Research, 178, 328–340.
Johnson, C. H., Patterson, A. D., Krausz, K. W., Lanz, C., Kang, D. W., Luecke, H., et al. (2011). Radiation metabolomics. 4. UPLC-ESI-QTOFMS-based metabolomics for urinary biomarker discovery in gamma-irradiated rats. Radiation Research, 175, 473–484.
Kermani, P., Leclerc, G., Martel, R., & Fareh, J. (2001). Effect of ionizing radiation on thymidine uptake, differentiation, and VEGFR2 receptor expression in endothelial cells: the role of VEGF(165). International Journal of Radiation Oncology Biology Physics, 50, 213–220.
Khan, A. R., Rana, P., Devi, M. M., Chaturvedi, S., Javed, S., Tripathi, R. P., et al. (2011). Nuclear magnetic resonance spectroscopy-based metabonomic investigation of biochemical effects in serum of gamma-irradiated mice. International Journal of Radiation Biology, 87, 91–97.
Kurohara, S. S., & Altman, K. I. (1962). Effect of exposure to ionizing radiation on creatine concentration in human and rat erythrocytes. Nature, 196, 151–153.
Lanz, C., Patterson, A. D., Slavik, J., Krausz, K. W., Ledermann, M., Gonzalez, F. J., et al. (2009). Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography-mass spectrometry combined with random forests machine learning algorithm. Radiation Research, 172, 198–212.
Lee, S. H., Jo, S. H., Lee, S. M., Koh, H. J., Song, H., Park, J. W., et al. (2004). Role of NADP+-dependent isocitrate dehydrogenase (NADP+-ICDH) on cellular defence against oxidative injury by gamma-rays. International Journal of Radiation Biology, 80, 635–642.
Lee, H. J., Lee, M., Kang, C. M., Jeoung, D., Bae, S., Cho, C. K., et al. (2007). Identification of possible candidate biomarkers for local or whole body radiation exposure in C57BL/6 mice. International Journal of Radiation Oncology Biology Physics, 69, 1272–1281.
Mak, T. D., Laiakis, E. C., Goudarzi, M., & Fornace, A. J, Jr. (2014). MetaboLyzer: A novel statistical workflow for analyzing postprocessed LC-MS metabolomics data. Analytical Chemistry, 86, 506–513.
Mansour, H. H. (2006). Protective role of carnitine ester against radiation-induced oxidative stress in rats. Pharmacological Research, 54, 165–171.
Noda, I. (2008). Scaling techniques to enhance two-dimensional correlation spectra. J Molecular Structure, 883, 216–227.
Ossetrova, N. I., Sandgren, D. J., Gallego, S., & Blakely, W. F. (2010). Combined approach of hematological biomarkers and plasma protein SAA for improvement of radiation dose assessment triage in biodosimetry applications. Health Physics, 98, 204–208.
Partridge, M. A., Chai, Y., Zhou, H., & Hei, T. K. (2010). High-throughput antibody-based assays to identify and quantify radiation-responsive protein biomarkers. International Journal of Radiation Biology, 86, 321–328.
Pearson, K. (1901). LIII. On lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 2, 559–572.
Plumb, R., Castro-Perez, J., Granger, J., Beattie, I., Joncour, K., & Wright, A. (2004). Ultra-performance liquid chromatography coupled to quadrupole-orthogonal time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry, 18, 2331–2337.
Porciani, S., Lanini, A., Balzi, M., Faraoni, P., & Becciolini, A. (2001). Polyamines as biochemical indicators of radiation injury. Phys Med, 17(Suppl 1), 187–188.
Randic, M., & Supek, Z. (1961). Urinary excretion of 5-hydroxyindolacetic acid after a single whole-body X-irradiation in normal and adrenalectomized rats. International Journal of Radiation Biology, 4, 151–153.
Reisz, J. A., Bansal, N., Qian, J., Zhao, W., & Furdui, C. M. (2014). Effects of ionizing radiation on biological molecules-mechanisms of damage and emerging methods of detection. Antioxidants & Redox Signaling, 21, 260–292.
Roux, A., Lison, D., Junot, C., & Heilier, J. F. (2011). Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology: A review. Clinical Biochemistry, 44, 119–135.
Sezen, O., Ertekin, M. V., Demircan, B., Karslioglu, I., Erdogan, F., Kocer, I., et al. (2008). Vitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced brain and retinal damages. Neurosurg Rev, 31, 205–213. discussion 213.
Takahashi, N., Boysen, G., Li, F., Li, Y., & Swenberg, J. A. (2007). Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney International, 71, 266–271.
Tang, X., Zheng, M., Zhang, Y., Fan, S., & Wang, C. (2013). Estimation value of plasma amino acid target analysis to the acute radiation injury early triage in the rat model. Metabolomics, 9, 853–863.
Tyburski, J. B., Patterson, A. D., Krausz, K. W., Slavik, J., Fornace, A. J. J., Gonzalez, F. J., et al. (2008). Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiation Research, 170, 1–14.
Tyburski, J. B., Patterson, A. D., Krausz, K. W., Slavik, J., Fornace, A. J, Jr, Gonzalez, F. J., et al. (2009). Radiation metabolomics. 2. Dose- and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice. Radiation Research, 172, 42–57.
Visser, W., Van Roermund, C., Ijlst, L., Waterham, H., & Wanders, R. (2007). Metabolite transport across the peroxisomal membrane. Biochemical Journal, 401, 365–375.
Zhang, Y., Zhou, X., Li, C., Wu, J., Kuo, J. E., & Wang, C. (2014). Assessment of early triage for acute radiation injury in rat model based on urinary amino acid target analysis. Molecular BioSystems, 10, 1441–1449.
Acknowledgments
This study was supported by the National Institute of Health (National Institute of Allergy and Infectious Diseases) Grant U19 A1067773. F.J.G. is supported by the National Cancer Instititue Intramural Research Program in the Center for Cancer Research. J.F.K. was supported in part by Grant DARPA-FY08-0004 from the Defense Advanced Research Projects Agency. The views expressed are those of the authors and do not reflect the official policy or position of the Armed Forces Radiobiology Research Institute, the Uniformed Services University, the Department of Defense, or the United States Government. The authors would like to thank Drs. Andrew D. Patterson (Penn. State Univ.) and David J. Brenner for helpful discussions and their support.
Conflict of interest
The authors have no conflicts of interest to report.
Ethical statement
All animal experiments were approved by the Armed Forces Radiobiology Research Institute’s Animal Care and Use Committee prior to initiation. Animals were maintained in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with the Guide for the Care and Use of Laboratory Animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tytus D. Mak and John B. Tyburski have contributed equally to this project.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mak, T.D., Tyburski, J.B., Krausz, K.W. et al. Exposure to ionizing radiation reveals global dose- and time-dependent changes in the urinary metabolome of rat. Metabolomics 11, 1082–1094 (2015). https://doi.org/10.1007/s11306-014-0765-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-014-0765-4