Abstract
Experimental samples are valuable and can represent a significant investment in time and resources. It is highly desirable at times to obtain as much information as possible from a single sample. This is especially relevant for systems biology approaches in which several ‘omics platforms are studied simultaneously. Unfortunately, each platform has a particular extraction methodology which increases sample number and sample volume requirements when multiple ‘omics are analyzed. We evaluated the integration of a yeast extraction method; specifically we explored whether fractions from a single metabolite extraction could be apportioned to multiple downstream ‘omics analytical platforms. In addition, we examined how variations to a chloroform/methanol yeast metabolite extraction regime influence metabolite recoveries. We show that protein suitable for proteomic analysis can be recovered from a metabolite extraction and that recovery of lipids, while reproducible, are not wholly quantitative. Higher quenching solution temperatures (−30 °C) can be used without significant leakage of intracellular metabolites when lower fermentation temperatures (20 °C) are employed. However, extended residence time in quenching solution, in combination with vigorous washing of quenched cell pellets, leads to extensive leakage of intracellular metabolites. Finally, there is minimal difference in metabolite amounts obtained when metabolite extractions are performed at 4 °C compared to extractions at −20 °C. The evaluated extraction method delivers material suitable for metabolomic and proteomic analyses from the same sample preparation.
Similar content being viewed by others
1 Introduction
The integration of reductionist techniques in the practice of systems biology (Kohl et al. 2010), into what has been described as ensemble descriptions of the cell (Nurse and Hayles 2011) has increased the diversity of analytical platforms used to obtain ‘omics datasets and has, in general, increased the number and type of extraction methodologies applied in any given experiment (for example see Oliver et al. 1998; Trauger et al. 2008; Weeks et al. 2006). Such an array of sample preparation methods not only increases experimental complexity but also the required amount of sample, which can be limited depending on the system under observation and experimental scale (Börner et al. 2007). Integration of ‘omics extraction methods, if possible, would have several advantages including reduced sample volume requirements facilitating high-resolution temporal sampling regimes for multiple platform analysis and simultaneous stabilization of transcriptome, proteome and metabolome. With metabolomic analyses being the most sensitive to variations in extraction conditions any attempt at integration might reasonably begin with metabolite extraction as a starting point.
Yeast metabolite extraction methods have been the subject of intense scrutiny and ongoing review since their initial development (Canelas et al. 2009; Canelas et al. 2008a; de Koning and van Dam 1992; Villas-Bôas et al. 2005; Villas-Bôas and Bruheim 2007; Wittmann et al. 2004; Hans et al. 2001). Metabolite extraction is fraught with the difficulty that poorly applied preparation methods can generate greater variation than originally exists within the biological sample itself. Only two methods, assessed by Canelas et al. (2009), have survived the rigor of the last decade to emerge as near complete, such that comprehensive metabolite extraction can be achieved with a degree of confidence (at least in yeast): boiling ethanol method (Gonzalez et al. 1997) and chloroform/methanol method (de Koning and van Dam 1992).
There are several advantages to the boiling ethanol method. It is rapid, requires minimal sample handling and it can be conducted in a small volume. Small volume of extracts can be rapidly concentrated by vacuum centrifugation. In contrast, chloroform/methanol extractions are more labor intensive and the relatively larger volume of metabolite-containing phases take longer to concentrate. However, the chloroform/methanol method has the promising feature that the extract is segregated into multiple fractions; a polar fraction (methanol/water), a non-polar fraction (chloroform) and a solvent insoluble fraction. Unlike the polar fraction, containing well characterized intracellular metabolites, the contents of the other fractions are only implied in earlier work evaluating yeast metabolite extraction; i.e. lipids in the non-polar fraction and proteins in the solvent insoluble fraction.
Similarities between the de Koning and van Dam (1992) metabolite extraction and the Folch et al. (1957) method of lipid extraction from fish have previously been noted (Villas-Bôas et al. 2005; Canelas et al. 2008a). The basic Folch method is generally robust and gives good recovery of all major lipid classes (Iverson et al. 2001). Given the similarities of the two methods, it is expected that the chloroform fraction of a de Koning and van Dam (1992) metabolite extraction will contain lipids, albeit extracted with varying degrees of efficiency. However, the methods have significant differences including extraction temperature, time of extraction, and the use of glass or zirconia beads to reliably extract lipids from yeast (Daum et al. 1999; Ejsing et al. 2009).
Recent work suggests that quantitative recovery of lipid species may be possible without the use of beads to first create a cell extract (Guan et al. 2010). In addition to lipids, the complete absence of fatty acids in the polar extracts of Villas-Bôas et al. (2005) suggests that the chloroform fraction may also extract this metabolite class. The extraction of lipids by a chloroform/methanol/water system is generally preferable to the use of ethanol, the alternative metabolite extraction method, due to the propensity of ethanol to oxidize to acetaldehyde, resulting in the formation of aldehyde adducts with amine containing lipids (Radin 1989).
The solvent insoluble material, following removal of liquid phases from a biphasic extraction, contains protein and polysaccharides. The protein component has been used as a crude means of evaluating initial biomass (de Koning and van Dam 1992; Villas-Bôas and Bruheim 2007). In these reports the quality of the protein component was not evaluated.
Traditionally, yeast protein extraction for use in proteomic work is achieved using a washing, lysis and precipitation regime. Washing removes components that have carried over from the growth medium, desalts the sample and, when performed with trichloroacetic acid, can assist with proteome stabilization (Horvath and Riezman 1994; Grassl et al. 2009; Wright et al. 1989). Protein extraction is commonly achieved using glass beads (Futcher et al. 1999; Wright et al. 1989; Conzelmann et al. 1988) or French press (Lee et al. 2011) followed by precipitation with acetone to further remove salts and prepare the sample for solublization in electrophoresis buffer (Picotti et al. 2009; Kümmel et al. 2010). Proteome stabilization and salt removal are key functions of all protein sample preparation methods.
Metabolite extractions also meet the requirements of protein extractions. Metabolite extractions are routinely performed under extremely low temperature conditions in order to stabilize the metabolome. Quenching has already been shown to stabilize the transcriptome (Pieterse et al. 2006; Martins et al. 2007) and is expected to do the same for the proteome. When biphasic extractions are employed, low temperatures are combined with the use of a denaturing solvent further enhancing proteome stability. In addition, a biphasic system can also quantitatively remove residual salt and other growth medium components which can interfere with downstream proteomic analyses (Wessel and Flügge 1984). Therefore, it is likely that the solvent insoluble material remaining after a metabolite extraction would contain protein suitable for more than biomass estimation. The converse; the use of protein extractions for the recovery of metabolites, is not so well matched. The use of acid as a stabilizing agent for proteins can lead to metabolite loss (Villas-Bôas et al. 2005) and protein extraction reagents are not, in general, compatible with direct use in mass spectrometry. Taken together these factors suggest a protocol in which metabolite extraction should be followed by protein extraction and not the other way around.
The rigorous evaluation of metabolite extraction methods to date has made clear the necessity of extraction method validation for each system to which it is applied. Therefore, this work explores how specific variations to the standard chloroform/methanol metabolite-extraction method for yeast, applied to a batch-fermentation, contribute to metabolite loss during the process of extraction. Specifically, the impact of quenching solution temperature, washing regime and extraction temperature were evaluated. In addition, we explored whether recovery of source material for proteomic and lipidomic work is possible from solvent insoluble and non-polar fractions respectively. Finally we propose an extraction method that delivers material suitable for both metabolomic and proteomic analyses from the same sample preparation.
2 Methods
2.1 Culture conditions
Saccharomyces cerevisiae strain AWRI1631 was obtained from the Australian Wine Research Institute Microorganism culture collection (WDCM22). Cultures were maintained on 1 % w/v yeast extract, 2 % w/v bacto peptone and 2 % w/v glycerol (YPG) agar plates at 28 °C. A progressive culturing regime, described below, from YPD to 50 % defined medium to 100 % defined medium was used to generate biomass for the inoculation of experimental cultures. This conditions the yeast to the defined medium environment and eliminates carry-over of complex medium. Overnight starter cultures were grown in 1 % w/v yeast extract, 2 % w/v bacto peptone and 2 % w/v d-glucose (YPD) liquid broth on a rotating wheel at 28 °C. YPD overnight cultures were used to inoculate 1:1 v/v defined medium:water, which were grown overnight in cotton-plugged Erlenmeyer flasks at 20 °C. Bioreactor vessels (New Brunswick Scientific, New Jersey, USA) were inoculated from 1:1 v/v defined medium:water overnight cultures to an optical density of 0.1 at 600 nm. Each bioreactor vessel contained 900 mL of defined medium that had been sparged with N2 gas until no further reduction in DO2 was observed.
Defined medium composition was as follows; d-glucose (20 gL−1), K2HPO4 (1.1 gL−1), MgSO4·7H2O (0.5 gL−1), CaCl2·2H2O (0.18 gL−1), NH4Cl (0.573 gL−1), trace minerals and vitamins as given in Schmidt et al. (2011) and made to a pH of 3.5 with potassium hydroxide. This minimal medium composition minimized the risk of interference by medium components during metabolite analysis of quenching solution and was used for all experiments except when the efficacy of washing steps was evaluated. For the evaluation of washing efficiency the growth medium was identical to that described above except that 100 gL−1 of both glucose and fructose was used. Fermentations were continued until residual sugar was no longer detectable (less than 0.1 gL−1). Glucose and fructose concentrations in fermentations and metabolite extracts were determined enzymatically (Randox Laboratories Ltd., Crumlin, UK) with modifications as described by Vermeir et al. (2007). Calibration curves relating glucose and fructose concentration to absorbance were fitted by least squares regression. The majority of work reported here used stationary phase cultures, sampled at a biomass concentration of 2.24 gL−1 dry cell weight (DCW), determined as described in (Liccioli et al. 2011). When efficacy of washing was being evaluated cultures were sampled during late-log phase growth from high sugar defined medium at a biomass concentration of 2.8 gL−1 (DCW).
2.2 Sampling of culture, quenching of metabolites and washing of biomass
A diagrammatic representation of the experimental workflow is shown in Fig. 1. A 10 mL sample containing 0.0224 g DCW was transferred from the fermenter vessel using an autosampler (Medicel, Helsinki, Finland) to 40 mL of 60 % v/v methanol in water (quenching solution) which had been pre-cooled, to either −30 °C (Q-30) or −40 °C (Q-40) in a Lauda ecoline RE120 (Lauda, Königshofen, Germany) with 60 % v/v ethylene glycol as cryo fluid and the addition of dry ice when required. The mean residence time of cell culture in sample tubing during sampling was less than 4 s. Different internal standard concentrations were used for quenching, washing and intracellular metabolite solutions such that their final concentrations were equivalent after sample drying and resuspension for analysis (see below). For the purpose of evaluating metabolite leakage during quenching, the quenching solution contained the following internal standards; adipic acid (0.04 μM), norvaline (0.5 μM) and sarcosine (0.5 μM). Yeast were collected immediately after quenching (Q-30 and Q-40) or after 20 min in quenching solution (Q-30T20) by centrifugation for 5 min at 1,780×g in a Hettich Universal 32R benchtop centrifuge (Tüttlingen, Germany) that had been pre-cooled to −20 °C. Quenching solution was decanted into fresh pre-weighed tubes and dried under nitrogen at 30 °C to facilitate methanol removal prior to using a centrifugal vacuum concentrator. The dried quenching solutions taken up in water (Q-40, Q-30 and Q-30T20) were used to evaluate leakage of intracellular metabolites.
Cell pellets were washed with −40 °C 60 % v/v methanol containing the following internal standards: adipic acid (0.4 μM), norvaline (5.0 μM) and sarcosine (5.0 μM). Two wash protocols were evaluated. Pellets were either vortexed in 5 mL of −40 °C wash solution and centrifuged as above (Vo) or rinsed by running 5 mL of wash solution around the rim of the tube with removal by decanting into fresh tubes (Ri). The rinsing procedure was performed twice on each sample. Washing solutions were dried using a centrifugal vacuum concentrator and taken up in water immediately prior to analysis. Washed cell pellets were extracted immediately (see below). Cell free medium samples (CF) were obtained by direct filtration as described by Mashego et al. (2003).
2.3 Extraction of metabolites
Extraction of intracellular metabolites from washed cells was performed using an adaptation of the methods described by de Koning and van Dam (1992). Yeast cell pellets isolated as described above were resuspended by vortexing for 30 s in 5 mL chloroform containing lysophosphatidylcholine (LPC, 0.2 μM) and cholesterol esters (CE, 0.2 μM) as internal standards for the non-polar fraction. Methanol (2.5 mL) was added to the chloroform suspended cells, mixed again by vortexing and the chloroform/methanol suspension transferred to a fresh, pre-weighed, 10 mL polypropylene centrifuge tube. Pre-cooled water (4 °C), containing 20 μM ribitol, 20 μM sarcosine, 1.5 μM adipic acid and 20 μM nor-valine as internal standards, was added to the chloroform/methanol suspension and mixed by shaking at 200 r.p.m. for 45 min at either −20 °C or 4 °C on a Ratek RM2 reciprocating mixer (Boronia, Victoria, Australia). The water/methanol and chloroform phases were separated by centrifugation for 5 min at 1,780×g and –20 °C. The upper water–methanol phase was removed (intracellular polar metabolite fraction—PM) leaving the non-polar lower phase (intracellular non-polar metabolite fraction—NpM) and protein containing interface. The chloroform lower phase was back extracted by the addition of a further 2 mL methanol and 2 mL water followed by vortexing for 30 s to create an emulsion and centrifuged as described above. The upper methanol/water phase was again removed and combined with the upper phase from the first centrifugation (PM). Methanol (5 mL) was added to the chloroform lower phase, samples were mixed by vortexing for 30 s and solvent insoluble material was precipitated by centrifugation at 1,780×g for 5 min at −20 °C. The supernatant was removed to a fresh pre-weighed tube (intracellular non-polar metabolite fraction—NpM). The pellet was washed with 5 mL methanol and again pelleted by centrifugation as described above. The methanol wash was discarded and the pellet was dried under a stream of N2 gas until just dry. Tubes were sealed and pellets were stored at −80 °C (solvent insoluble fraction—SIF). Intracellular polar (PM) and non-polar (NpM) metabolite fractions were dried using a centrifugal vacuum concentrator (Labconco, Kansas city, USA.) as recommended by Villas-Bôas et al. (2005). Dried PM and NpM fractions were taken up in water and butanol:methanol (50:50) respectively prior to analysis.
A monophasic lipid extraction method as described previously (Bligh and Dyer 1959) was used for comparison. Briefly, 2 mL of chloroform were added to quenched and washed cells and vortexed to resuspend the pellet. Then 4 mL of methanol and 2 mL of water were added and the cells were extracted by shaking at shaking at 200 r.p.m. for 45 min on a Ratek RM2 reciprocating mixer at either 4 °C (ME4) or 20 °C (ME20).
2.4 Protein solublization from yeast pellets and solvent extracted material
Proteins were extracted from solvent insoluble fractions (SIF) as follows; 1 mL of 2D buffer (7 M urea, 2 M thiourea, 20 mM tris and 4 % w/v 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)) was added to SIF fractions, probe sonicated for 2 × 10 s on ice using a probe sonicator (Branson sonifier 450, John Morris Scientific, Chatswood, Australia) and then water bath sonication (Transsonic 700/H, Elma GMBH, Singen, Germany) for 15 min. The supernatant (SIFE1) was collected by centrifuging at 20,000×g for 10 min and pelleted material was re-extraction as above (SIFE2). Both SIFE1 and SIFE2 were pooled, desalted and concentrated by using 3 kDa cut off concentrators (Sartorius AG, Goettingen, Germany). Proteins were also extracted directly from washed yeast cell pellets using the method of Herbert et al. (2006) to create a reference protein extract (Ref Ex). Following protein extraction, supernatants were precipitated by addition of 9 volumes of acetone, incubation at −20 °C for 1 h and centrifugation at 4,000×g for 15 min. Pellets were solubilized with 7 M urea, 2 M thiourea, 4 % CHAPS and 20 mM Tris. For 2-D gel electrophoresis, conductivity was kept at, or below 300 μScm−1, measured using TwinCond conductivity meter B-173 (Horiba, Kyoto, Japan). Protein concentrations were determined with a Bradford protein assay kit (Sigma, St Louis, USA) using BSA as a standard.
2.5 Separation of protein fraction by 2-D electrophoresis
Prior to protein separation by 2-D electrophoresis, extracted proteins were reduced with 5 mM Tributyl phosphine (TBP) and alkylated with 15 mM acrylamide for 90 min at room temperature. Following reduction and alkylation, 100 μg of protein were loaded on a 17 cm pH 5–8 linear IPG strip (Bio-Rad, Regents Park, Australia). First dimension isoelectric focusing was performed on an Ettan IPGphor II (GE Health Care, Uppsala, Sweden) for ~20 h at 20 °C using the following program: 300 V for 4 h, linear increase from 300 to 8,000 V over 8 h and hold at 8,000 V until approximately 100 kVh had been accumulated. The focused IPG strip was equilibrated for approximately 2 × 15 min in equilibration buffer (6 M urea, 2 % v/v SDS, 20 % w/v glycerol, 0.375 M tris–HCl buffer). Equilibrated IPG strips were overlaid onto 8–18 % w/v SDS-polyacrylamide gradient gel (180 mm × 190 mm) and sealed with 0.5 % w/v agarose solution containing 0.1 % w/v bromophenol blue (Bio-Rad, Regents Park, Australia). The SDS-polyacrylamide gradient gels were cast in our laboratory using ProteanII xi Multi-Gel Casting Chamber (Bio-Rad, Regents Park, Australia) according to manufacture’s instructions. Molecular weight standards (Bio-Rad, Regents Park, Australia) were loaded at the edge of the cast gradient gel. IEF separated proteins were then resolved in the second dimension at 5 mA/gel overnight followed by 40 mA/gel with a constant temperature of 4 °C until the bromophenol blue dye front had just run off the bottom of the gel. The gel was fixed in 10 % v/v methanol and 7 % v/v acetic acid for 4 h and stained with SYPRO® Ruby staining solution at room temperature overnight. The gel was then destained twice with 10 % methanol and 7 % acetic acid for 4 h and 1× with 1 % v/v acetic acid. Stained gels were imaged at 100 μm resolution using a Typhoon Trio 9,400 variable mode laser scanner (GE Life Sciences, Rydalmere, Australia) with 457 nm excitation and 610 nm BP 30 emission filter.
2.6 Protein identification
Protein spots on 2-D gel were excised using an ExQuest Robotic fluorescent spot cutter equipped with a CCD camera and 1 mm diameter cutting head (Bio-Rad, Regents Park, Australia). The gel plugs were washed 3 times with 25 mM ammonium bicarbonate in 50 % v/v acetonitrile (ACN) for 15 min at 37 °C with gentle agitation, dehydrated with 100 % ACN for 10 min at room temperature and incubated at 37 °C until the gel plugs were completely dry. Dry gel plugs were rehydrated with trypsin solution (5 ng/μL, Sigma, St Louis, USA) for 1 h at 4 °C. The excess trypsin solution was removed and 25 mM ammonium bicarbonate was added to cover the gel plugs which were incubated overnight at 37 °C for digestion. Tryptic digestion was quenched by adding 0.1 % trifluoroacetic acid (TFA) and peptides were extracted from gel plugs by water bath sonication (Transsonic 700/H, Elma GMBH, Singen, Germany). The peptides were desalted and concentrated using Perfect Pure C18 zip-tip (Eppendorf, North Ryde, Australia) and were spotted onto a Matrix-assisted laser desorption/ionization (MALDI) sample plate with 1 μL of matrix (α-cyano-4-hydroxycinnamic acid, 4 mg mL−1 in 90 % v/v ACN, 0.1 % v/v TFA) and allowed to air dry.
MALDI mass spectrometry was performed with an Applied Biosystems 4,800 Plus MALDI TOF/TOF™ Analyser (Carlsbad, USA). A neodymium-doped yttrium aluminium garnet laser (355 nm) was used to irradiate the sample. Spectra were acquired in reflectron mode in the mass range 700–3,500 Da and were externally calibrated using known peptide standards (bradykinin, neurotensin, angiotensin and adrenocorticotropic hormone). The eight strongest peptides from the MS scan were isolated and fragmented in tandem time of flight mode (by collision-induced dissociation using filtered laboratory air), then re-accelerated to measure their masses and intensities. A near point calibration was applied to give a typical mass accuracy better than 50 ppm. The peptide peak list was used to interrogate the Mascot database (Matrix Science Ltd, London, UK). All samples were searched against Saccharomyces cerevisiae (baker’s yeast) in SwissProt with the following search parameters; type of search: PMF and MSMS, enzyme: trypsin, variable modifications: methyl (DE), oxidation (M), propionamide (C), mass values: monoisotopic, protein mass: unrestricted, peptide mass tolerance: ±50 ppm, fragment mass tolerance: ±0.8 Da, peptide charge state: 1+ , max missed cleavages: 1). High MOWSE scores in the database search indicated a likely match, confirmed by manual inspection. Percentage sequence coverage, the difference between calculated and observed peptide masses, the number of missed cleavages was taken into account for positive identification in addition to how well the MW and pI of the identified protein matched other experimental data such as 2-DE.
2.7 Quantification of intracellular and extracellular metabolites
γ-Aminobutyric acid and 20 proteinogenic amino acids in the cell extract as well as the quenching solution, washing solutions and the culture broth were quantified using high performance liquid chromatography (HPLC). The protocol employed precolumn o-phtalaldehyde derivatization followed by fluorescence detection and is described elsewhere (Dietmair et al. 2010). Norvaline and sarcosine were used as internal standards for primary and secondary amines, respectively. The concentration of internal standard in all samples was 250 μM. This was achieved by appropriate concentration/dilution steps.
Analyses of 32 intracellular metabolites (18 glycolysis and TCA intermediaries, 11 nucleotides phosphate and 3 nicotinamide adenine dinucleotides) were conducted on a Dionex UltiMate 3,000 liquid chromatography system (Thermo Fischer, Scoresby, Australia) coupled to an ABSciex 4,000 QTRAP mass spectrometer (Waverly, Australia). Chromatographic separation was achieved on a Gemini-NX C18 150 × 2 mm I.D., 3 μm, 110 Å particle column (Phenomenex, Aschaffenburg, Germany) operated at 55 °C. Mobile phase adapted from Luo et al. (2007) was 7.5 mM tributylamine aqueous solution adjusted to pH 4.95 with glacial acetic acid (eluent A) and acetonitrile (eluent B). The linear HPLC gradient profile was 0 % B for 8 min, 0–20 % B for 12 min, 20–27 % B for 10 min, 27–100 % B for 1 min, followed by a column wash at 100 % B for 3 min and re-equilibration back to initial conditions for 16 min, all at 0.3 mL min−1. Injection volumes of 10 μL were used. Mobile phase was directly introduced into the mass spectrometer.
The mass spectrometer was equipped with a TurboV electrospray source operated in negative ion mode. Infusions of analyte standards dissolved in purified water were used for tuning compound dependent MS parameters. Declustering potential (DP), collision energy (CE) and collision cell exit potential (CXP) of each transition were optimized and are shown in Online Resource 1. Entrance potential (EP) was fixed to −10 V for all transitions. To tune source dependent parameters an analyte standard mix was infused along with mobile phase (7.5 mM tributylamine : acetonitrile 50:50 (v/v), 100 μL/min) connected by a T-port. A combination of parameters was chosen based on highest abundance for the majority of analytes. These optimized parameters were ionspray voltage −4,500 V, nebulizer (GS1), auxiliary (GS2), curtain (CUR) and collision (CAD) gases were 60, 60, 20 and medium (arbitrary units), respectively, being generated from pressurized air in a N300DR nitrogen generator (Peak Scientific, Massachusetts, USA). The auxiliary gas temperature was maintained at 350 °C. To obtain adequate selectivity and sensitivity, the mass spectrometer was set to unit resolution and scheduled Multiple Reaction Monitoring mode after determining the retention time of each analyte under the chromatographic conditions described above. The acquisitions were made with a Target Scan Time setting of 1 s. Peaks varied in width with the narrowest peak being 0.3 min at the baseline. During acquisition, the number of points acquired across this particular peak was 18. For peaks with a greater peak width, the number of points defining the peak would increase.
Calibration curves were constructed by plotting peak area of the analyte against the concentration of the compound from reference standards. The relative concentration of each metabolite in the cell extract sample was determined by back-calculating the metabolite peak area against the calibration curve.
2.8 Quantification of cellular lipids
Dried chloroform or monophasic cellular extracts were resuspended in butanol/methanol (1:1, v/v) containing 5 μM ammonium formate. Cellular lipids were separated by injecting 5 μL aliquots onto a 50 mm × 2.1 mm × 2.7 μm Ascentis Express RP Amide column (Supelco, Sigma, St Louis, USA) at 35 °C using an Agilent LC 1,200 (Mulgrave, Australia). Lipids were eluted at 0.2 mL min−1 over a 5 min gradient of water/methanol/tetrahydrofuran (50:20:30, v/v/v) to water/methanol/tetrahydrofuran (5:20:75, v/v/v), with the final buffer held for 3 min. Lipids were analysed by electrospray ionisation-mass spectrometry (ESI-MS) using an Agilent Triple Quad 6,460 (Mulgrave, Australia). Lipid species from each lipid class were identified using precursor ion scanning from 100 to 1,000 m/z, in positive ion mode, phosphatidylcholines (PC, precursors of m/z 184.1), sphingomyelins (SM, m/z 184.1), ceramides (CER, m/z 264.6), cholesterol esters (CE, m/z 369.4), phosphatidylglycerols (PG, m/z 189) and in negative ion mode phosphatidylinositols (PI, m/z 241). Neutral loss scanning was used to identify phosphatidylethanolamines (PE, in positive ion mode, neutral loss of m/z 141) and phosphatidylserines (PS, negative ion mode, m/z 87). Identified lipid species were quantified using multiple reaction monitoring (MRM) with a 50 ms dwell time for the simultaneous measurements of ~20–50 compounds and the chromatographic peak width of 30–45 s, a minimum data points collected across the peak was 12 to 16. Optimised parameters for capillary, fragmentor, and collision voltages were 4,000 V, 140–380, and 15–60 V, respectively. In all cases, the collision gas was nitrogen at 7 L min−1. ESI-MS data was processed using Agilent Mass Hunter (Mulgrave, Australia). Lipid standards (Avanti Polar Lipids, Alabaster, USA) were prepared by combining equal volumes of individual lipid stock solutions. The standard solution was then diluted to provide a set of calibration solutions ranging in concentration from 0.1 to 10 μM. Calibration curves were constructed by least squares linear regression, fitting reverse phase peak area of the analyte against the concentration of the lipid in the reference standards. The concentration of each lipid species in the cell extract sample was estimated by normalizing the lipid peak area against the internal standard (LPC) and then using the regression model to convert normalized peak area to lipid concentration. Detected lipid species, 55 in total from 8 lipid classes, were annotated as follows; lipid class (sum of carbon atoms in the two fatty acid chains:sum of double bonds in the fatty acid chains).
2.9 Statistical methods
All samples were taken from the same bioreactor vessel (no biological replication). Each treatment or extraction condition (i.e. quenching at −30 or −40 °C) was performed in triplicate (3 technical replicates for each treatment). A 2-tailed pairwise t test at p < 0.05 was used to make comparisons of amounts obtained between two treatments. Comparison of more than two treatments was made using ANOVA (p < 0.05). Dunnet’s multiple comparison post test was applied when multiple comparisons were made to a control sample. All results are expressed as mean ± standard deviation. Statistical analyses were performed using Graphpad PRISM (GraphPad Software Inc. LaJolla, CA, USA). The degree of difference between treatments was explored by calculating the percent difference (% diff = ([m 1 − m 2]/[(m 1 + m 2)/2)]) × 100) where m = mean metabolite concentration.
3 Results and discussion
3.1 Effect of quenching solution temperature on metabolite leakage
Evaluations of quenching solution temperature by a number of laboratories have indicated that sub –40 °C temperatures are necessary in order to limit wholesale leakage of intracellular metabolites during sample preparation. This temperature requirement is based, in part, on the maximal permissible temperature of the quenching solution following addition of a warm sample, such that leakage of intracellular metabolites is minimized. In two comprehensive evaluations of quenching conditions (Canelas et al. 2008a; Villas-Bôas et al. 2005) the cultures being evaluated were grown at 30 °C which, once added to quenching solution, resulted in temperatures at around −10 °C. We routinely perform fermentations at 20 °C. Therefore we tested whether lower sample temperatures permitted the use of higher quenching solution temperatures.
Quenching solution temperature following addition of 20 °C sample did not exceed −17 °C irrespective of whether the quenching solution was −30 °C or −40 °C. Nanomole recoveries of amino acids from the sub −40 °C quenched sample (Q-40) were compared with all other treatments and amino acids recovered from cell free media (CF). The higher quenching solution temperature (Q-30) did not result in significantly increased leakage of amino acids into the quenching solution (Table 1). The low level of leakage observed agreed with some reports (Gonzalez et al. 1997; Bolten and Wittmann 2008) but differed to some degree with others (Canelas et al. 2008a; Tredwell et al. 2011). The losses of glutamate (>50 %) reported by Canelas et al. (2008a) or the losses of alanine, tyrosine and isoleucine reported by Tredwell et al. (2011) in otherwise identical conditions were not observed in this study. Extensive leakage of amino acids occurred during 20 min incubation at −30 °C (Q-30T20), demonstrating the importance of sample processing soon after quenching.
3.2 Choice of yeast cell washing regime can increase metabolite leakage
Washing of biomass is a critical and at times overlooked component of metabolite preparation. It is especially important when analysis of batch cultures or cultures growing in complex media is being undertaken, not only because leakage into the washing solution can contribute to variations in metabolite estimation but because medium carryover can interfere with post extraction analytical methods. Two cell pellet washing procedures are described in reports in which the washing step is explicitly given: vortexing of the pellet in cold methanol followed by centrifugation (Castrillo et al. 2003) or methanol addition to the pellet and decanting (Canelas et al. 2008a). Canelas et al. (2008a) reported that a small but significant proportion of metabolites could be recovered from the washing solution.
The above two washing regimes (vortexing and rinsing) were evaluated following quenching at −30 °C. A significant increase in amino acid leakage into wash solution was observed when cells were washed by vortexing rather than rinsing (Table 2). The efficiency of washing, i.e. how well each washing regime removed medium components, was also evaluated. This was achieved through a separate extraction of early exponential phase cells growing in defined medium containing 165 gL−1 of glucose and fructose at the time of sampling. Measures of culture medium removal efficiency by each washing regime were based on the recovery of glucose and fructose in the polar phase of a biphasic extraction. Intracellular metabolite extracts from cells that had been washed by vortexing had 2.5 fold less sugar than when cells were washed by rinsing (Table 2). Despite the higher sugar concentrations in metabolite extracts of ‘rinsed’ cells, rinsing still achieved an 850-fold reduction in medium component concentrations, which ultimately did not interfere with mass spectral analysis of intracellular metabolites.
3.3 Metabolite extraction temperature does not effect estimation of intracellular metabolite concentrations
Biphasic extraction of metabolites from yeast usually involves extended shaking at −20 °C (de Koning and van Dam 1992) or more recently at −40 °C (Canelas et al. 2009). Such cryo-extractions require specialized equipment or extensive modification of existing facilities. Extractions at 4 °C have been reported (Fairchild et al. 2010; t’Kindt et al. 2009) but the effect of this extraction temperature on metabolite recoveries were not compared to lower temperature extractions. We assessed if the use of higher extraction temperature compromised the recovery of intracellular metabolites from yeast.
The effect of temperature on the extraction of 53 intracellular metabolites was evaluated; nanomole amounts obtained from extractions performed at either 4 °C or −20 °C of quenched and washed yeast cell pellets are reported in Table 3. For the majority of metabolites the amounts obtained were indistinguishable, irrespective of extraction temperature. Of the 8 metabolites that were significantly different, we observed increased amounts of ATP, GTP, CTP, UTP, GDP, malate and E4P but decreased amounts of NADH and FBP when extractions were performed at 4 °C. Percent differences were greater than 15 % for nine metabolites, most notably NADH (−51 %) and GTP (42 %). NADH and FBP have previously been shown to be unstable during extraction (Klimacek et al. 2010). However, instability does not explain the increased amounts obtained from 4 °C extractions of all the nucleoside triphosphates, which were also shown to be unstable in the same study. An alternative explanation for the decreased quantities of NADH and FBP obtained during extraction at 4 °C is that these differences reflect both measurement variation and comparatively small intracellular concentrations. The large increase in GTP obtained by extraction at 4 °C is consistent with increased amounts of other nucleoside triphosphates and point towards either more favourable conditions for the extraction of nucleoside triphosphates or metabolite conversion due to residual enzymatic activity.
Mean metabolite specific yields expressed as μmol/gDCW are given in the last column of Table 3 in order to compare with the work of others. While direct comparison is difficult due to the differing experimental circumstances (such as strain, growth medium composition, oxygen status, growth phase, etc.) the recoveries of intracellular metabolites presented in Table 3 are, in general, consistent with the intracellular metabolite concentrations in S. cerevisiae estimated using varying extraction methods (Canelas et al. 2008b; Canelas et al. 2009; Gonzalez et al. 1997).
3.4 Overall contributions of sample preparation method to metabolite loss
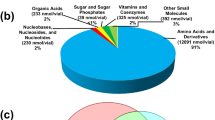
Figure 2 shows total amino acid amounts obtains from all stages of the metabolite processing pipeline. Less than 2 % of the total recoverable molar amount of each amino acid was lost during quenching regardless of the quenching temperature. After a 20 min incubation in quenching solution amino acid leakage into the quenching solution increased from an average of 2 % to between 10 and 20 % for many of the amino acids in Table 1. The increase in metabolite leakage with increasing residence time in quenching solution shows that time in quenching solution is of significantly greater importance than quenching solution temperature, at least when lower culture temperatures are used. Increased metabolite leakage was also reported in a study exploring differences in extraction of intracellular metabolites following extended incubation in quenching solution at different temperatures (Villas-Bôas and Bruheim 2007).
Contribution of different processing methods to total loss of metabolites during extraction from S. cerevisiae. The molar amounts of amino acids obtained from quenching solution (Q), washing solution (Vo and Ri) and metabolite extract (EX) were summed and the total number of moles obtained for each process combination was regarded as 100 %. The molar amount obtained individually from quenching solution, washing solution and polar extract is then expressed as a percentage of that total. (a) Quenched at −30 °C, washed by vortexing and extracted at 4 °C (Q-30:Vo:EX4), (b) Quenched at −30 °C, washed by rinsing and extracted at 4 °C (Q-30:Ri:EX4), (c) Quenched at −30 °C for 20 min, washed by vortexing and extracted at 4 °C (Q-30T20:Vo:EX4)
Leakage of amino acids during a vortex wash resulted in an additional average loss of 2 % of the total. Leakage of glycine (6.8 %) and alanine (5.2 %) into wash solution were higher than leakage of other amino acids and represented significant fractions of the total amounts obtained of those amino acids. Four of the top five amino acids with the greatest loss during washing correspond to amino acids with low molecular weights (less than 120 Da) indicating that loss of small metabolites could be exacerbated by the use of a vortex washing regime. Losses incurred during both quenching and washing were on average less than 1.5 % (max 3.9 %) when rapid quenching was combined with washing by rinsing (Fig. 2b). Therefore, if washing is required and if the residual concentration of medium components can be accommodated in the analytical platform used for intracellular metabolite analysis, then it would be preferable to rinse cell pellets at least twice rather than using the more vigorous vortex and centrifugation method. If extensive cell pellet washing were to be combined with an extended residence time in quenching solution, extensive leakage of intracellular metabolites should be anticipated (Fig. 2c).
3.5 Biphasic extraction yields protein suitable for proteomic analysis
Standard yeast protein extraction protocols for proteomic analysis generally utilize TCA or thermal pre-treatment in order to stabilize proteins prior to storage and extraction (Grassl et al. 2009) or extract directly into lysis buffer containing protease inhibitors and denaturants (Futcher et al. 1999). The quenching step of metabolite preparation should effectively stabilize the proteome in preparation for extraction. The subsequent biphasic extraction removes medium components that may interfere with subsequent electrophoretic analysis or proteolytic treatments as discussed by Wessel and Flügge (1984). That protein for use in proteomic work can be derived using sequential extraction methodologies has been shown for systems other than yeast (Weckwerth et al. 2004). We evaluated the quality of protein in metabolite extracted insoluble material using our preferred extraction method (sample quenching at −30 °C, rinsing of the cell pellet and metabolite extraction at 4 °C) by comparison with a reference protein extraction method.
Figure 3 shows the equivalence of general protein profiles between (a) protein extracted using a reference protein extraction method (Herbert et al. 2006) and (b) protein from metabolite extracted yeast. One of the features of the metabolite extraction method described in this work is centrifugation at 1,575×g as opposed to centrifugation at 10,000×g that is commonly used to isolate protein. Comparison of the two gels in Fig. 3 shows no additional recovery of small molecular weight proteins or alteration in overall protein profile when a higher centrifugation speed is used. We did observe an increased overall amount of protein extracted per gram DCW using the reference method (data not shown).
Comparison of protein recoveries from yeast cultures at different growth phases Protein from stationary phase cultures was extracted from whole cells (a) using the method of Herbert et al. (2006), and from solvent insoluble material remaining following a biphasic chloroform/methanol extraction of metabolites (b) as described in the materials and methods. Extracted proteins were separated in the first dimension using pH 5–8 immobilized gradient isoelectric focusing strips and in the second dimension by 8–16 % SDS-PAGE. Separated proteins were stained with Sypro ruby
Subsequently, proteome stability was evaluated using 2-D PAGE (Fig. 4) and MALDI-MS/MS. Resolution in 2D-PAGE stained with sypro ruby was comparable with previous reports (Grassl et al. 2009). One hundred and ninety protein spots were excised and identified using MALDI MS/MS. We observed little evidence of large scale degradation, with most proteins running in accordance with their predicted molecular weights (Online Resource 2). Only 9 spots were identified as proteins whose predicted molecular weights were higher than was observed on the gel. Spots 8 and 9 were identified as VMA1, a protein known to undergo post-translational intein-mediated splicing to generate a 69 kDa subunit of the vacuolar adenosine triphosphatase (Kane et al. 1990), which concurs with the observed molecular weight. Spot 164 was identified as CCP1, a mitochondrial protein that undergoes processing to remove a mitochondrial import signal resulting in a 33 kDa mature protein (Maccecchini et al. 1979), also in accordance with observation. Fragments of Eno1 (spot 181), Eno2 (spots 179, 180) and SSA2 (spots 168 and 169) were detected at sizes that could not be explained by cellular protein processing events and were detected at very low abundance, indicating limited degradation of those proteins (circled, Fig. 3). However, we cannot rule out that these fragments arise as a result of normal protein turnover rather than artifacts of sample isolation. This protein survey demonstrates that solvent insoluble material remaining after a biphasic metabolite extraction of yeast is capable of yielding intact protein of a quality suitable for proteomic work.
2-D PAGE separation of proteins recovered from solvent-insoluble material. Protein was solubilized from solvent-insoluble material following a biphasic metabolite extraction of a stationary phase culture. Separated proteins were stained with Sypro-ruby and the identity of individual spots (numbered) was determined by MALDI-MS/MS. Highlighted numbers indicate protein identifications that do not correspond to predicted molecular weights
3.6 Effectiveness of lipid extraction is dependent on extraction temperature
It has been suggested that the lower chloroform fraction may be of use for the evaluation of lipid metabolism (implied in Canelas et al. 2009; Smart et al. 2010). Although it has previously been shown that lipid recovery can be highly variable when using the Folch method without prior cell disruption (Hanson and Lester 1980) we evaluated lipid recoveries from biphasic metabolite extractions (BPE) of stationary phase yeast, at −20 °C or 4 °C compared with monophasic Bligh and Dyer (1959) extractions (ME) at 4 °C or 20 °C.
There were no significant differences in amounts obtained from biphasic extractions performed at either −20 °C (BPE-20) or 4 °C (BPE4) of either whole lipid classes (Online Resource 3) or individual lipid species (Online Resource 4), with the exception of PC(26:0), PC(26:1) and PC(38:1). BPE4 resulted in slightly higher amounts of these lipid species. Monophasic extraction at 4 °C (ME4) gave significantly lower amounts of total LPG, PC and PE lipid classes but significantly higher amounts of total PI and PS than BPE4. This pattern of recovery was true for all species within each class. When monophasic extractions were performed at 20 °C, amounts of total LPC, LPG, PC, PE and PG lipid classes were no different than from BPE4 extractions. However, total amounts obtained of PI, PS and ergosterol were significantly higher than for any other extraction condition including ME4. These lipid classes are both apolar and polar and individually represent significant proportions of total cellular lipid. Much of the additional lipid was recovered during a second extraction step that was performed as part of the ME protocol (data not shown).
A broad range of lipids were recovered from the chloroform phase of a biphasic extraction, which is commonly used for the preparation of intracellular metabolites from yeast. However, yields of individual lipids can vary with extraction temperature and extraction method. Specifically, extraction at 20 °C favoured recovery of serine, inositol and sterol-based lipids. Additional optimization will be required before quantitative extraction of lipids is achieved from an integrated metabolite extraction protocol. Re-extraction with the same solvent system is beneficial and, in combination with the use of increasingly hydrophobic solvents as outlined by Guan et al. (2010), may provide the necessary improvement in lipid recoveries.
4 Concluding remarks
Experimental samples are valuable and can represent a significant investment in time and resources. It is therefore desirable to extract as much information as possible from a single experiment. Specifically, volume limitation either due to culture miniaturization or multiple sequential sampling from batch systems, can lead to situations where additional biomass for parallel ‘omics extractions is not viable. For example, microtiter plate cultures provide enough cell biomass for a single metabolite extraction but unfortunately there is not enough biological material left for other analyses. Additionally, sequential sampling from individual batch cultures in order to derive temporal ‘omic datasets can decrease dramatically residual volume and therefore affect growth kinetics and cell metabolism. This is exaggerated at early time point samples, when biomass concentration is low and large sample volumes are required. We have shown that additional value can be obtained from yeast metabolite extractions through the recovery of material from fractions that are often discarded.
Solvent insoluble material recovered following a standard biphasic chloroform/methanol extraction of polar metabolites contains protein of a quality that is suitable for proteomic analysis. Solubilization in an appropriate buffer can deliver protein preparations for either electrophoretic or mass spectral proteomic work. The metabolite extraction procedure has the additional advantage that the proteome is rapidly stabilized through cold and chloroform denaturation and that interfering environmental contaminants are removed. As expected the chloroform fraction obtained during this procedure contained lipids that could be quantitatively and reproducibly recovered. However, overall lipid amounts were significantly less compared with lipids extracted using a monophasic system at higher temperature. It is possible that repeated extractions of insoluble material at low temperature may lead to increased recoveries.
A comprehensive characterization of the yeast metabolite extraction method reported here, which was performed at higher temperatures than usually recommended, revealed minimal metabolite leakage during quenching. It is likely that this was the result of the lower fermentation temperature. However, considerable metabolite leakage was observed when quenched cells were vigorously washed, even when very cold washing solution was used. Therefore, washing of yeast cells is generally counter productive and should not be undertaken in situations when the analytical platform can withstand the associated introduction of environmental material. Even when very high concentrations of environmental components are present a simple rinse of tube walls is generally sufficient to reduce contaminating components to levels compatible with mass spectral analyses.
Finally, very little difference was found in metabolite amounts obtained from extractions performed at either 4 °C or −20 °C. Even in those instances when differences were statistically significant it is not entirely clear that the magnitude of those differences were biologically meaningful. Based on our results metabolite extraction at 4 °C is sufficient both qualitatively and quantitatively when facilities for very low temperature extractions are not available.
References
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917.
Bolten, C. J., & Wittmann, C. (2008). Appropriate sampling for intracellular amino acid analysis in five phylogenetically different yeasts. Biotechnology Letters, 30(11), 1993–2000.
Börner, J., Buchinger, S., & Schomburg, D. (2007). A high-throughput method for microbial metabolome analysis using gas chromatography/mass spectrometry. Analytical Biochemistry, 367(2), 143–151.
Canelas, A. B., Ras, C., ten Pierick, A., van Dam, J. C., Heijnen, J. J., & Van Gulik, W. M. (2008a). Leakage-free rapid quenching technique for yeast metabolomics. Metabolomics, 4(3), 226–239.
Canelas, A. B., ten Pierick, A., Ras, C., Seifar, R. M., van Dam, J. C., van Gulik, W. M., et al. (2009). Quantitative evaluation of intracellular metabolite extraction techniques for yeast metabolomics. Analytical Chemistry, 81(17), 7379–7389.
Canelas, A. B., van Gulik, W. M., & Heijnen, J. J. (2008b). Determination of the cytosolic free NAD/NADH ratio in Saccharomyces cerevisiae under steady-state and highly dynamic conditions. Biotechnology and Bioengineering, 100(4), 734–743.
Castrillo, J. I., Hayes, A., Mohammed, S., Gaskell, S. J., & Oliver, S. G. (2003). An optimized protocol for metabolome analysis in yeast using direct infusion electrospray mass spectrometry. Phytochemistry, 62(6), 929–937.
Conzelmann, A., Riezman, H., Desponds, C., & Bron, C. (1988). A major 125 kD membrane glycoprotein of Saccharomyces cerevisiae is attached to the lipid bilayer through and inositol-containing phospholipid. EMBO Journal, 7(7), 2233–2240.
de Koning, W., & van Dam, K. (1992). A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Analytical Biochemistry, 204(1), 118–123.
Daum, G., Tuller, G., Nemec, T., Hrastnik, C., Balliano, G., Cattel, L., et al. (1999). Systematic analysis of yeast strains with possible defects in lipid metabolism. Yeast, 15(7), 601–614.
Dietmair, S., Timmins, N. E., Gray, P. P., Nielsen, L. K., & Kroemer, J. O. (2010). Towards quantitative metabolomics of mammalian cells: Development of a metabolite extraction protocol. Analytical Biochemistry, 404(2), 155–164.
Ejsing, C. S., Sampaio, J. L., Surendranath, V., Duchoslav, E., Ekroos, K., Klemm, R. W., et al. (2009). Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proceedings of the National academy of Sciences of the United States of America, 106(7), 2136–2141.
Fairchild, J. N., Horvath, K., Gooding, J. R., Campagna, S. R., & Guiochon, G. (2010). Two-dimensional liquid chromatography/mass spectrometry/mass spectrometry separation of water-soluble metabolites. Journal of Chromatography A, 1217(52), 8161–8166.
Folch, J., Lees, M., & Sloane-Stanley, G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry, 226(1), 497–509.
Futcher, B., Latter, G. I., Monardo, P., McLaughlin, C. S., & Garrels, J. I. (1999). A sampling of the yeast proteome. Molecular and Cellular Biology, 19(11), 7357–7368.
Gonzalez, B., Francois, J., & Renaud, M. (1997). A rapid and reliable method for metabolite extraction in yeast using boiling buffered ethanol. Yeast, 13(14), 1347–1355.
Grassl, J., Westbrook, J. A., Robinson, A., Boren, M., Dunn, M. J., & Clyne, R. K. (2009). Preserving the yeast proteome from sample degradation. Proteomics, 9(20), 4616–4626.
Guan, X. L., Riezman, I., Wenk, M. R., & Riezman, H. (2010). Yeast lipid analysis and quantification by mass spectrometry. Methods in Enzymology, 470, 369–391.
Hans, M. A., Heinzle, E., & Wittmann, C. (2001). Quantification of intracellular amino acids in batch cultures of Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 56(5–6), 776–779.
Hanson, B. A., & Lester, R. L. (1980). The extraction of inositol-containing phospholipids and phophatidylcholine from Saccharomyces cerevisiae and Neurospora crassa. Journal of Lipid Research, 21(3), 309–315.
Herbert, B. R., Grinyer, J., McCarthy, J. T., Isaacs, M., Harry, E. J., Nevalainen, H., et al. (2006). Improved 2-DE of microorganisms after acidic extraction. Electrophoresis, 27(8), 1630–1640.
Horvath, A., & Riezman, H. (1994). Rapid protein extraction from Saccharomyces cerevisiae. Yeast, 10(10), 1305–1310.
Iverson, S. J., Lang, S. L. C., & Cooper, M. H. (2001). Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids, 36(11), 1283–1287.
Kane, P. M., Yamashiro, C. T., Wolczyk, D. F., Neff, N., Goebl, M., & Stevens, T. H. (1990). Protein splicing converts the yeast TFP1 gene-product to the 69 kD subunit of the vacuolar H+-adenosine triphosphatase. Science, 250(4981), 651–657.
Klimacek, M., Krahulec, S., Sauer, U., & Nidetzky, B. (2010). Limitations in xylose-fermenting Saccharomyces cerevisiae, made evident through comprehensive metabolite profiling and thermodynamic analysis. Applied and Environmental Microbiology, 76(22), 7566–7574.
Kohl, P., Crampin, E. J., Quinn, T. A., & Noble, D. (2010). Systems biology: An approach. Clinical Pharmacology and Therapeutics, 88(1), 25–33.
Kümmel, A., Ewald, J. C., Fendt, S.-M., Jol, S. J., Picotti, P., Aebersold, R., et al. (2010). Differential glucose repression in common yeast strains in response to HXK2 deletion. FEMS Yeast Research, 10(3), 322–332.
Lee, M. V., Topper, S. E., Hubler, S. L., Hose, J., Wenger, C. D., Coon, J. J., et al. (2011). A dynamic model of proteome changes reveals new roles for transcript alteration in yeast. Molecular Systems Biology, 7, 514.
Liccioli, T., Tran, T. M. T., Cozzolino, D., Jiranek, V., Chambers, P. J., & Schmidt, S. A. (2011). Microvinification-how small can we go? Applied Microbiology and Biotechnology, 89(5), 1621–1628.
Luo, B., Groenke, K., Takors, R., Wandrey, C., & Oldiges, M. (2007). Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography-mass spectrometry. Journal of Chromatography A, 1147(2), 153–164.
Maccecchini, M. L., Rudin, Y., & Schatz, G. (1979). Transport of proteins across the mitochondrial outer-membrane; A precursor form of the cytoplasmically made intermembrane enzyme cytochrome c peroxidase. Journal of Biological Chemistry, 254(16), 7468–7471.
Martins, A. M., Sha, W., Evans, C., Martino-Catt, S., Mendes, P., & Shulaev, V. (2007). Comparison of sampling techniques for parallel analysis of transcript and metabolite levels in Saccharomyces cerevisiae. Yeast, 24(3), 181–188.
Mashego, M. R., van Gulik, W. M., Vinke, J. L., & Heijnen, J. J. (2003). Critical evaluation of sampling techniques for residual glucose determination in carbon-limited chemostat culture of Saccharomyces cerevisiae. Biotechnology and Bioengineering, 83(4), 395–399.
Nurse, P., & Hayles, J. (2011). The cell in an era of systems biology. Cell, 144(6), 850–854.
Oliver, S. G., Winson, M. K., Kell, D. B., & Baganz, F. (1998). Systematic functional analysis of the yeast genome. Trends in Biotechnology, 16(9), 373–378.
Picotti, P., Bodenmiller, B., Mueller, L. N., Domon, B., & Aebersold, R. (2009). Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell, 138(4), 795–806.
Pieterse, B., Jellema, R. H., & van der Werf, M. J. (2006). Quenching of microbial samples for increased reliability of microarray data. Journal of Microbiological Methods, 64(2), 207–216.
Radin, N. S. (1989). Lipid extraction. In A. A. Boulton, G. B. Baker, & L. A. Horrocks (Eds.), Lipids and related compounds (1 ed., Vol. 7, pp. 1–61, Neuromethods). Clifton, NJ: Humana Press.
Schmidt, S. A., Dillon, S., Kolouchova, R., Henschke, P. A., & Chambers, P. J. (2011). Impacts of variations in elemental nutrient concentration of Chardonnay musts on Saccharomyces cerevisiae fermentation kinetics and wine composition. Applied Microbiology and Biotechnology, 91(2), 365–375.
Smart, K. F., Aggio, R. B. M., Van Houtte, J. R., & Villas-Boas, S. G. (2010). Analytical platform for metabolome analysis of microbial cells using methyl chloroformate derivatization followed by gas chromatography-mass spectrometry. Nature Protocols, 5(10), 1709–1729.
t’Kindt, R., Morreel, K., Deforce, D., Boerjan, W., & Van Bocxlaer, J. (2009). Joint GC-MS and LC-MS platforms for comprehensive plant metabolomics: Repeatability and sample pre-treatment. Journal of Chromatography B, 877(29), 3572–3580.
Trauger, S. A., Kalisak, E., Kalisiak, J., Morita, H., Weinberg, M. V., Menon, A. L., et al. (2008). Correlating the transcriptome, proteome, and metabolome in the environmental adaptation of a hyperthermophile. Journal of Proteome Research, 7(3), 1027–1035.
Tredwell, G. D., Edwards-Jones, B., Leak, D. J., & Bundy, J. G. (2011). The development of metabolomic sampling procedures for Pichia pastoris, and baseline metabolome data. PLoS ONE, 6(1), e16286.
Vermeir, S., Nicolai, B. M., Jans, K., Maes, G., & Lammertyn, J. (2007). High-throughput microplate enzymatic assays for fast sugar and acid quantification in apple and tomato. Journal of Agricultural and Food Chemistry, 55(9), 3240–3248.
Villas-Bôas, S. G., & Bruheim, P. (2007). Cold glycerol-saline: The promising quenching solution for accurate intracellular metabolite analysis of microbial cells. Analytical Biochemistry, 370, 87–97.
Villas-Bôas, S. G., Højer-Pedersen, J., Åkesson, M., Smedsgaard, J., & Nielsen, J. (2005). Global metabolite analysis of yeast: evaluation of sample preparation methods. Yeast, 22(14), 1155–1169.
Weckwerth, W., Wenzel, K., & Fiehn, O. (2004). Process for the integrated extraction identification, and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics, 4(1), 78–83.
Weeks, M. E., Sinclair, J., Butt, A., Chung, Y.-L., Worthington, J. L., Wilkinson, C. R. M., et al. (2006). A parallel proteomic and metabolomic analysis of the hydrogen peroxide- and Sty1p-dependent stress response in Schizosaccharomyces pombe. Proteomics, 6(9), 2772–2796.
Wessel, D., & Flügge, U. I. (1984). A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Analytical Biochemistry, 138(1), 141–143.
Wittmann, C., Krömer, J. O., Kiefer, P., Binz, T., & Heinzle, E. (2004). Impact of the cold shock phenomenon on quantification of intracellular metabolites in bacteria. Analytical Biochemistry, 327(1), 135–139.
Wright, A. P. H., Bruns, M., & Hartley, B. S. (1989). Extraction and rapid inactivation of proteins from Saccharomyces cerevisiae by trichloroacetic acid precipitation. Yeast, 5(1), 51–53.
Acknowledgments
This project was supported by Australia’s grape growers and winemakers through their investment body the Grape and Wine Research and Development Corporation, with matching funds from the Australian Government. The AWRI is part of the Wine Innovation Cluster, Adelaide, South Australia. Systems Biology research at the AWRI is performed using resources provided by Bioplatforms Australia as part of the National Collaborative Research Infrastructure Strategy, an initiative of the Australian Government, in addition to funds from the South Australian State Government. We would like to acknowledge Paul Chambers for his review of the manuscript and Wade Hines and Nathan Watson-Haigh for their input during manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, S.A., Jacob, S.S., Ahn, S.B. et al. Two strings to the systems biology bow: co-extracting the metabolome and proteome of yeast. Metabolomics 9, 173–188 (2013). https://doi.org/10.1007/s11306-012-0437-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-012-0437-1