Abstract
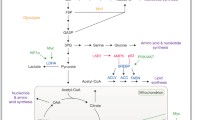
In this present study, the efficacy of metabolomics as a tool for tumor cell energetics for in vitro cell cultures was demonstrated with full competence for the first time by elucidating the anabolic and energy-yielding segments of glycolysis and glutaminolysis, which constitute a part of energy metabolism in tumor cells. By synchronizing colon cancer cells SW480 and SW620 in culture, the metabolome specific to cell cycle phases was analyzed using nuclear magnetic resonance spectroscopy. At the G1/S transition of the cell cycle (i.e. transition from cell growth to duplication of genetic material), the majority of the energy production was realized by glycolysis through a high channeling of glucose carbons towards lactate. During the late S phase, the majority of energy was produced by glutaminolysis through a high channeling of glutamine carbons towards lactate, while the glucose carbons were channeled towards bio-synthetic pathways. These results indicate that the metabolism of proliferating cells is heterogeneous throughout the cell cycle and can be better interpreted on the basis of different cell cycle phases. These findings could be exploited for the development of a tool for tumor diagnosis as well as for targeting tumors.








Similar content being viewed by others
References
Aledo, J. C., Segura, J. A., Medina, M. A., Alonso, F. J., De Castro, I. N., & Marquez, J. (1994). Phosphate-activated glutaminase expression during tumor development. FEBS Letters, 341, 39–42.
Bar, J., Cohen-Noyman, E., Geiger, B., & Oren, M. (2004). Attenuation of the p53 response to DNA damage by high cell density. Oncogene, 23, 2128–2137.
Bereiter-Hahn, J., Munnich, A., & Woiteneck, P. (1998). Dependence of energy metabolism on the density of cells in culture. Cell Structure and Function, 23, 85–93.
Chang, J. M., Lee, H. J., Goo, J. M., Lee, H. Y., Lee, J. J., Chung, J. K., et al. (2006). False positive and false negative FDG-PET scans in various thoracic diseases. Korean Journal of Radiology, 7, 57–69.
Coquelle, A., Mouhamad, S., Pequignot, M. O., Braun, T., Carvalho, G., Vivet, S., et al. (2006). Cell cycle-dependent cytotoxic and cytostatic effects of bortezomib on colon carcinoma cells. Cell Death and Differentiation, 13, 873–875.
Czernin, J., & Phelps, M. E. (2002). Positron emission tomography scanning: Current and future applications. Annual Review of Medicine, 53, 89–112.
de Anta, J. M., REAL, F. X., & MAYOL, X. (2005). Low tumor cell density environment yields survival advantage of tumor cells exposed to MTX in vitro. Biochimica Et Biophysica Acta-General Subjects, 1721, 98–106.
Forshed, J., Torgrip, R. J. O., Åberg, K. M., Karlberg, B., Lindberg, J., & Jacobsson, S. P. (2005). A comparison of methods for alignment of NMR peaks in the context of cluster analysis. Journal of Pharmaceutical and Biomedical Analysis, 38, 824–832.
Frederiks, W. M., van Marle, J., van Oven, C., Comin-Anduix, B., & Cascante, M. (2006). Improved localization of glucose-6-phosphate dehydrogenase activity in cells with 5-cyano-2,3-ditolyl-tetrazolium chloride as fluorescent redox dye reveals its cell cycle-dependent regulation. Journal of Histochemistry and Cytochemistry, 54, 47–52.
Harper, J. V. (2004). Synchronization of cell populations in G1/S and G2/M phases of the cell cycle. In T. Humphrey and G. Brooks (Eds.), Cell cycle control: Mechanisms and protocols. Totowa: Humana Press.
Hyland, P. L., Keegan, A. L., Curran, M. D., Middleton, D., Mckenna, P. G., & Barnett, Y. A. (2000). Effect of a dCTP:dTTP pool imbalance on DNA replication fidelity in Friend murine erythroleukemia cells. Environmental and Molecular Mutagenesis, 36, 87–96.
Jackowski, S. (1994). Coordination of membrane phospholipid-synthesis with the cell-cycle. Journal of Biological Chemistry, 269, 3858–3867.
Jana, S., & Blaufox, M. D. (2006). Nuclear medicine studies of the prostate, testes, and bladder. Seminars in Nuclear Medicine, 36, 51–72.
Junker, B. H., Klukas, C., & Schreiber, F. (2006). VANTED: A system for advanced data analysis and visualization in the context of biological networks. BMC Bioinformatics, 7, 109.
Kennady, P. K., Ormerod, M. G., Singh, S., & Pande, G. (2004). Variation of mitochondrial size during the cell cycle: A multiparameter flow cytometric and microscopic study. Cytometry Part A, 62A, 97–108.
Koc, A., Wheeler, L. J., Mathews, C. K., & Merrill, G. F. (2004). Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. Journal of Biological Chemistry, 279, 223–230.
Lassen, U., Daugaard, G., Eigtved, A., Damgaard, K., & Friberg, L. (1999). 18F-FDG whole body positron emission tomography (PET) in patients with unknown primary tumours (UPT). European Journal of Cancer, 35, 1076–1082.
Liu, Y. (2006). Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostatic Diseases, 9, 230–234.
Margineantu, D. H., Cox, W. G., Sundell, L., Sherwood, S. W., Beechem, J. A., & Capaldi, R. A. (2002). Cell cycle dependent morphology changes and associated mitochondrial DNA redistribution in mitochondria of human cell lines. Mitochondrion, 1, 425–435.
Martínez-Bisbal, M. C., Luis, M. B., José, P., Antonio, R., Pilar, F., José, L. L., et al. (2004). 1H and 13C HR-MAS spectroscopy of intact biopsy samples ex vivo and in vivo 1H MRS study of human high grade gliomas. NMR in Biomedicine, 17, 191–205.
Masquelier, M., & Vitols, S. (2004). Drastic effect of cell density on the cytotoxicity of daunorubicin and cytosine arabinoside. Biochemical Pharmacology, 67, 1639–1646.
Mazurek, S., & Eigenbrodt, E. (2003). The tumor metabolome. Anticancer Research, 23, 1149–1154.
Mazurek, S., Zwerschke, W., Jansen-Durr, P., & Eigenbrodt, E. (2001). Metabolic cooperation between different oncogenes during cell transformation: Interaction between activated ras and HPV-16 E7. Oncogene, 20, 6891–6898.
Mckeehan, W. L. (1982). Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep, 6, 635–650.
Moreadith, R. W., & Lehninger, A. L. (1984). The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem, 259, 6215–6221.
O’conner, P. M., & Jackman, J. (1995). Synchronization of mammalian cells. In M. Pagano (Ed.), Cell cycle: Materials and methods. New York: Springer-Verlag.
Parlo, R. A., & Coleman, P. S. (1984). Enhanced rate of citrate export from cholesterol-rich hepatoma mitochondria. The truncated Krebs cycle and other metabolic ramifications of mitochondrial membrane cholesterol. Journal of Biological Chemistry, 259, 9997–10003.
Pauwels, E. K., Sturm, E. J., Bombardieri, E., Cleton, F. J., & Stokkel, M. P. (2000). Positron-emission tomography with [18F]fluorodeoxyglucose. Part I. Biochemical uptake mechanism and its implication for clinical studies. Journal of Cancer Research and Clinical Oncology, 126, 549–559.
Pfeiffer, T., Schuster, S., & Bonhoeffer, S. (2001). Cooperation and competition in the evolution of ATP-producing pathways. Science, 292, 504–507.
Poulin, N. M., Matthews, J. B., Skov, K. A., & Palcic, B. (1994). Effects of fixation method on image cytometric measurement of DNA content and distribution in cells stained for fluorescence with propidium iodide. The Joumal of Histochemistry and Cytochemistry, 42, 1149–1156.
Rodrigues, M., Chehne, F., Kalinowska, W., Berghammer, P., Zielinski, C., & Sinzinger, H. (2000). Uptake of 99mTc-MIBI and 99mTc-tetrofosmin into malignant versus nonmalignant breast cell lines. Journal of Nuclear Medicine, 41, 1495–1499.
Rossignol, R., Gilkerson, R., Aggeler, R., Yamagata, K., Remington, S. J., & Capaldi, R. A. (2004). Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Research, 64, 985–993.
Schiepers, C., & Hoh, C. K. (1998). Positron emission tomography as a diagnostic tool in oncology. European Radiology, 8, 1481–1494.
Seemann, M. D. (2004). PET/CT: Fundamental principles. European Journal of Medical Research, 9, 241–246.
Toyoda, M., Sakuragawa, N., Arai, Y., Yoshikawa, H., Sugai, K., Arima, M., et al. (1989). Positron emission tomography using pyruvate-1-11C in two cases of mitochondrial encephalomyopathy. Annals of Nuclear Medicine, 3, 103–109.
Usadel, B., Nagel, A., Thimm, O., Redestig, H., Blaesing, O. E., Palacios-Rojas, N., et al. (2005). Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiology, 138, 1195–1204.
Valverde, D., Quintero, M. R., Candiota, A. P., Badiella, L., Cabanas, M. E., & Arus, C. (2006). Analysis of the changes in the H-1 NMR spectral pattern of perchloric acid extracts of C6 cells with growth. NMR in Biomedicine, 19, 223–230.
Vukelic, Z., & Kalanj-Bognar, S. (2001). Cell density-dependent changes of glycosphingolipid biosynthesis in cultured human skin fibroblasts. Glycoconjugate Journal, 18, 429–437.
Warburg, O. (1925). über den Stoffwechsel der Carcinomzelle. Journal of Molecular Medicine, 4, 534–536.
Warburg, O. (1956). On the origin of cancer cells. Science, 123, 309–314.
Zhu, X., Kumar, R., Mandal, M., Sharma, N., Sharma, H. W., Dhingra, U., et al. (1996). Cell cycle-dependent modulation of telomerase activity in tumor cells. Proceedings of the National Academy of Sciences, 93, 6091–6095.
Zielke, H. R., Sumbilla, C. M., Sevdalian, D. A., Hawkins, R. L., & Ozand, P. T. (1980). Lactate: a major product of glutamine metabolism by human diploid fibroblasts. Journal of Cellular Physiology, 104, 433–441.
Zielke, H. R., Zielke, C. L., & Ozand, P. T. (1984). Glutamine: A major energy source for cultured mammalian cells. Federation Proceedings, 43, 121–125.
Acknowledgments
The financial support from the Ministerium für Innovation, Wissenschaft, Forschung und Technologie des Landes Nordrhein-Westfalen; Bundesministerium für Bildung und Forschung (Pakt für Forschung und Innovation, dem gemeinsamen Förderungsprogramm von Bund und Ländern) is greatly acknowledged. The author is also thankful to EU-NMR-Centre in Birmingham and Ulrich Günther for the NMR measurements and to Jorg Lambert and Roland Hergenröder for their fruitful discussions at ISAS-Institute for Analytical Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. SI-1
Comparison of asynchronous and synchronous samples of SW480 cells. Fold change pattern of 61 metabolites, containing 51 significantly (p < 0.01) regulated metabolites. Asynchronous (white), G1/S transition phase (light gray), S phase (gray) and G2/M transition phase (black – not relevant to this paper) (PNG 89 kb) (PNG 90 kb)
Rights and permissions
About this article
Cite this article
Maddula, S., Baumbach, J.I. Heterogeneity in tumor cell energetic metabolome at different cell cycle phases of human colon cancer cell lines. Metabolomics 7, 509–523 (2011). https://doi.org/10.1007/s11306-010-0267-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-010-0267-y