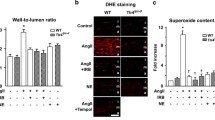
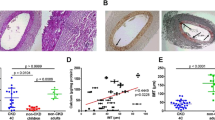
Abstract
Ectonucleoside triphosphate diphosphohydrolase-1, the major vascular/immune ectonucleotidase, exerts anti-thrombotic and immunomodulatory actions by hydrolyzing extracellular nucleotides (danger signals). Hypertension is characterized by vascular wall remodeling, endothelial dysfunction, and immune infiltration. Here our aim was to investigate the impact of arterial hypertension on CD39 expression and activity in mice. Arterial expression of CD39 was determined by reverse transcription quantitative real-time PCR in experimental models of hypertension, including angiotensin II (AngII)-treated mice (1 mg/kg/day, 21 days), deoxycorticosterone acetate-salt mice (1% salt and uninephrectomy, 21 days), and spontaneously hypertensive rats. A decrease in CD39 expression occurred in the resistance and conductance arteries of hypertensive animals with no effect on lymphoid organs. In AngII-treated mice, a decrease in CD39 protein levels (Western blot) was corroborated by reduced arterial nucleotidase activity, as evaluated by fluorescent (etheno)-ADP hydrolysis. Moreover, serum-soluble ADPase activity, supported by CD39, was significantly decreased in AngII-treated mice. Experiments were conducted in vitro on vascular cells to determine the elements underlying this downregulation. We found that CD39 transcription was reduced by proinflammatory cytokines interleukin (IL)-1β and tumor necrosis factor alpha on vascular smooth muscle cells and by IL-6 and anti-inflammatory and profibrotic cytokine transforming growth factor beta 1 on endothelial cells. In addition, CD39 expression was downregulated by mechanical stretch on vascular cells. Arterial expression and activity of CD39 were decreased in hypertension as a result of both a proinflammatory environment and mechanical strain exerted on vascular cells. Reduced ectonucleotidase activity may alter the vascular condition, thus enhancing arterial damage, remodeling, or thrombotic events.





Similar content being viewed by others
Abbreviations
- ADO:
-
Adenosine
- ADP:
-
Adenosine 5′-diphosphate
- AMP:
-
Adenosine 5′-monophosphate
- ATP:
-
Adenosine 5′-triphosphate
- AngII:
-
Angiotensin II
- DOCA:
-
Deoxycorticosterone acetate
- EC:
-
Endothelial cell
- IL:
-
Interleukin
- IFN-γ:
-
Interferon gamma
- MRA:
-
Mesenteric resistance artery
- SHR:
-
Spontaneously hypertensive rat
- NTPDase:
-
Nucleoside triphosphate diphosphohydrolase
- RT-qPCR:
-
Reverse transcription quantitative real-time PCR
- TGF-β1:
-
Transforming growth factor beta 1
- TNF-α:
-
Tumor necrosis factor alpha
- VSMC:
-
Vascular smooth muscle cell
References
Idzko M, Ferrari D, Riegel AK, Eltzschig HK (2014) Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 124(7):1029–1037. https://doi.org/10.1182/blood-2013-09-402560
Longhi MS et al. (2017) Purinergic signaling during intestinal inflammation. J Mol Med (Berl). 95(9):915–925. https://doi.org/10.1007/s00109-017-1545-1
Burnstock G (2017) Purinergic signaling in the cardiovascular system. Circ Res 120(1):207–228. https://doi.org/10.1161/CIRCRESAHA.116.309726
Ledderose C, Bao Y, Kondo Y, Fakhari M, Slubowski C, Zhang J, Junger WG (2016) Purinergic signaling and the immune response in sepsis: a review. Clin Ther 38(5):1054–1065. https://doi.org/10.1016/j.clinthera.2016.04.002
Marcus AJ, Broekman MJ, Drosopoulos JHF, Olson KE, Islam N, Pinsky DJ, Levi R (2005) Role of CD39 (NTPDase-1) in thromboregulation, cerebroprotection, and cardioprotection. Semin Thromb Hemost 31(2):234–246. https://doi.org/10.1055/s-2005-869528
Eltzschig HK, Sitkovsky MV, Robson SC (2013) Purinergic signaling during inflammation. N Engl J Med 368(13):1260. https://doi.org/10.1056/NEJMc1300259
Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198(5):783–796. https://doi.org/10.1084/jem.20030891
Kohler D, Eckle T, Faigle M, Grenz A, Mittelbronn M, Laucher S, Hart ML, Robson SC, Muller CE, Eltzschig HK (2007) CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116(16):1784–1794. https://doi.org/10.1161/CIRCULATIONAHA.107.690180
Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orléans-Juste P, Marceau F, Thorin É, Sévigny J (2010) NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res 85(1):204–213. https://doi.org/10.1093/cvr/cvp265
Kauffenstein G, Fürstenau CR, D'Orléans-Juste P, Sévigny J (2010) The ecto-nucleotidase NTPDase1 differentially regulates P2Y1 and P2Y2 receptor-dependent vasorelaxation. Br J Pharmacol 159(3):576–585. https://doi.org/10.1111/j.1476-5381.2009.00566.x
Lee MY, Griendling KK (2008) Redox signaling, vascular function, and hypertension. Antioxid Redox Signal 10(6):1045–1059. https://doi.org/10.1089/ars.2007.1986
Intengan HD, Schiffrin EL (2001) Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38(3 Pt 2):581–587. https://doi.org/10.1161/hy09t1.096249
McMaster WG, Kirabo A, Madhur MS, Harrison DG (2015) Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116(6):1022–1033. https://doi.org/10.1161/CIRCRESAHA.116.303697
Schiffrin EL (2014) Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 126(4):267–274. https://doi.org/10.1042/CS20130407
Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BMW (2007) Renin-angiotensin system and cardiovascular risk. Lancet 369(9568):1208–1219. https://doi.org/10.1016/S0140-6736(07)60242-6
Kanthi Y, Hyman MC, Liao H, Baek AE, Visovatti SH, Sutton NR, Goonewardena SN, Neral MK, Jo H, Pinsky DJ (2015) Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis. J Clin Invest 125(8):3027–3036. https://doi.org/10.1172/JCI79514
Grote K et al (2003) Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92(11):e80–e86. https://doi.org/10.1161/01.RES.0000077044.60138.7C
Warner TD, Mitchell JA (2003) HIF, stretching to get control of VEGF. Clin Sci (Lond) 105(4):393–394
Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y (2013) Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112(11):1466–1478. https://doi.org/10.1161/CIRCRESAHA.111.300166
Burnstock G (2002) Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol 22(3):364–373. https://doi.org/10.1161/hq0302.105360
Kauffenstein G, Tamareille S, Prunier F, Roy C, Ayer A, Toutain B, Billaud M, Isakson BE, Grimaud L, Loufrani L, Rousseau P, Abraham P, Procaccio V, Monyer H, de Wit C, Boeynaems JM, Robaye B, Kwak BR, Henrion D (2016) Central role of P2Y6 UDP receptor in arteriolar myogenic tone. Arterioscler Thromb Vasc Biol 36(8):1598–1606. https://doi.org/10.1161/ATVBAHA.116.307739
Nishimura A, Sunggip C, Tozaki-Saitoh H, Shimauchi T, Numaga-Tomita T, Hirano K, Ide T, Boeynaems JM, Kurose H, Tsuda M, Robaye B, Inoue K, Nishida M (2016) Purinergic P2Y6 receptors heterodimerize with angiotensin AT1 receptors to promote angiotensin II-induced hypertension. Sci Signal 9(411):ra7. https://doi.org/10.1126/scisignal.aac9187
Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N (2012) P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303(8):F1207–F1215. https://doi.org/10.1152/ajprenal.00051.2012
Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR (1997) The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest 99(6):1351–1360. https://doi.org/10.1172/JCI119294
Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD (1999) Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5(9):1010–1017. https://doi.org/10.1038/12447
Sun X, Cardenas A, Wu Y, Enjyoji K, Robson SC (2009) Vascular stasis, intestinal hemorrhage, and heightened vascular permeability complicate acute portal hypertension in cd39-null mice. Am J Physiol Gastrointest Liver Physiol 297(2):G306–G311. https://doi.org/10.1152/ajpgi.90703.2008
Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, Bach FH (1997) Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med 185(1):153–163. https://doi.org/10.1084/jem.185.1.153
Mercier N, Kiviniemi TO, Saraste A, Miiluniemi M, Silvola J, Jalkanen S, Yegutkin GG (2012) Impaired ATP-induced coronary blood flow and diminished aortic NTPDase activity precede lesion formation in apolipoprotein E-deficient mice. Am J Pathol 180(1):419–428. https://doi.org/10.1016/j.ajpath.2011.10.002
Lecka J, Bloch-Boguslawska E, Molski S, Komoszynski M (2010) Extracellular purine metabolism in blood vessels (part II): activity of ecto-enzymes in blood vessels of patients with abdominal aortic aneurysm. Clin Appl Thromb Hemost 16(6):650–657. https://doi.org/10.1177/1076029609354329
Krotz F et al (2002) Depolarization of endothelial cells enhances platelet aggregation through oxidative inactivation of endothelial NTPDase. Arterioscler Thromb Vasc Biol 22(12):2003–2009. https://doi.org/10.1161/01.ATV.0000043454.08172.51
Barbaro NR et al (2015) Vascular damage in resistant hypertension: TNF-alpha inhibition effects on endothelial cells. Biomed Res Int 2015:631594
Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH, Dumont AS (2013) TNF-alpha induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab 33(10):1564–1573. https://doi.org/10.1038/jcbfm.2013.109
Justin Rucker A, Crowley SD (2017) The role of macrophages in hypertension and its complications. Pflugers Arch 469(3–4):419–430. https://doi.org/10.1007/s00424-017-1950-x
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176(7):3877–3883. https://doi.org/10.4049/jimmunol.176.7.3877
Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A 105(23):8067–8072. https://doi.org/10.1073/pnas.0709684105
Levesque SA et al (2010) NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol 40(5):1473–1485. https://doi.org/10.1002/eji.200939741
Li P, Li Yl, Li Zy, Wu Yn, Zhang Cc, A X, Wang Cx, Shi Ht, Hui Mz, Xie B, Ahmed M, Du J (2014) Cross talk between vascular smooth muscle cells and monocytes through interleukin-1beta/interleukin-18 signaling promotes vein graft thickening. Arterioscler Thromb Vasc Biol 34(9):2001–2011. https://doi.org/10.1161/ATVBAHA.113.303145
Lee DL, Sturgis LC, Labazi H, Osborne JB Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW (2006) Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290(3):H935–H940. https://doi.org/10.1152/ajpheart.00708.2005
Thiolat A, Semerano L, Pers YM, Biton J, Lemeiter D, Portales P, Quentin J, Jorgensen C, Decker P, Boissier MC, Louis-Plence P, Bessis N (2014) Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol 66(2):273–283. https://doi.org/10.1002/art.38246
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170(6):1807–1816. https://doi.org/10.2353/ajpath.2007.070112
Piera-Velazquez S, Jimenez SA (2012) Molecular mechanisms of endothelial to mesenchymal cell transition (EndoMT) in experimentally induced fibrotic diseases. Fibrogenesis Tissue Repair 5(Suppl 1):S7. https://doi.org/10.1186/1755-1536-5-S1-S7
Lu D, Insel PA (2014) Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis. Am J Physiol Cell Physiol 306(9):C779–C788. https://doi.org/10.1152/ajpcell.00381.2013
Shyu KG (2009) Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin Sci (Lond) 116(5):377–389. https://doi.org/10.1042/CS20080163
Moeckel D, Jeong SS, Sun X, Broekman MJ, Nguyen A, Drosopoulos JHF, Marcus AJ, Robson SC, Chen R, Abendschein D (2014) Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Transl Med 6(248):248ra105. https://doi.org/10.1126/scitranslmed.3009246
Guckelberger O, Sun XF, Sévigny J, Imai M, Kaczmarek E, Enjyoji K, Kruskal JB, Robson SC (2004) Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost 91(3):576–586. https://doi.org/10.1160/TH03-06-0373
McRae JL et al (2013) Overexpression of CD39 protects in a mouse model of preeclampsia. Nephrology (Carlton) 18(5):351–355
Visovatti SH, Hyman MC, Goonewardena SN, Anyanwu AC, Kanthi Y, Robichaud P, Wang J, Petrovic-Djergovic D, Rattan R, Burant CF, Pinsky DJ (2016) Purinergic dysregulation in pulmonary hypertension. Am J Physiol Heart Circ Physiol 311(1):H286–H298. https://doi.org/10.1152/ajpheart.00572.2015
d'Almeida SM et al (2016) The ecto-ATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancer tumor-associated macrophages: regulatory role of IL-27. Oncoimmunology 5(7):e1178025. https://doi.org/10.1080/2162402X.2016.1178025
Hot A, Lavocat F, Lenief V, Miossec P (2013) Simvastatin inhibits the pro-inflammatory and pro-thrombotic effects of IL-17 and TNF-alpha on endothelial cells. Ann Rheum Dis 72(5):754–760. https://doi.org/10.1136/annrheumdis-2012-201887
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783(5):673–94. https://doi.org/10.1016/j.bbamcr.2008.01.024
Jalkanen J, Yegutkin GG, Hollmén M, Aalto K, Kiviniemi T, Salomaa V, Jalkanen S, Hakovirta H (2015) Aberrant circulating levels of purinergic signaling markers are associated with several key aspects of peripheral atherosclerosis and thrombosis. Circ Res 116(7):1206–1215. https://doi.org/10.1161/CIRCRESAHA.116.305715
Acknowledgments
The authors thank Dimitri Bréard for expert HPLC technical assistance.
JS received support from the Canadian Institutes of Health Research (CIHR) and was also the recipient of a “Chercheur National” Scholarship from the Fonds de Recherche du Québec – Santé (FRQS).
Funding
MitoVasc Institute was supported by INSERM, CNRS, University of Angers, CHU of Angers, Région Pays de la Loire, Angers-Loire Métropole, and Département du Maine et Loire. CR was supported by grants from INSERM and Région Pays de la Loire.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Charlotte Roy declares that she has no conflict of interest.
Julie Tabiasco declares that she has no conflict of interest.
Antoine Caillon declares that he has no conflict of interest.
Yves Delneste declares that he has no conflict of interest.
Jean Merot declares that he has no conflict of interest.
Julie Favre declares that she has no conflict of interest.
Anne Laure Guihot declares that she has no conflict of interest.
Ludovic Martin declares that he has no conflict of interest.
Daniele Nascimento declares that she has no conflict of interest.
Bernhard Ryffel declares that he has no conflict of interest.
Simon C. Robson declares that he has no conflict of interest.
Jean Sévigny declares that he has no conflict of interest.
Daniel Henrion declares that he has no conflict of interest.
Gilles Kauffenstein declares that he has no conflict of interest.
Ethical approval
Animals were manipulated in accordance with European Community Standards on the Care and Use of Laboratory Animals (authorization No. 6422).
Electronic supplementary material
Fig. S1
Arterial expression of NTPDase family members as an effect of hypertension. NTPDase 1, 2, 3, and 8 mRNA expression was determined in thoracic aorta of sham and AngII-infused mice (n = 5) by RT-qPCR. Data are presented as means ± SEM. **p < 0.01 (Student’s t-test) vs. controls. (PDF 43.9 kb)
Fig. S2
ADPase activity in mouse abdominal aortic homogenates. ADPase activity was measured by HPLC with fluorescence etheno-ADP as a substrate as described in methods. Entpd1+/− mice display a 45% reduction in ADPase activity compared to wild-type (1.82 ± 0.2 vs. 3.3 ± 0.3 nmol/μg protein/h). More than 90% ADPase activity is lost in Entpd1−/− mice compared to wild-type activity (0.24 ± 0.02 vs. 3.3 ± 0.3 nmol/μg/h). These results confirmed a 50% reduction in CD39 expression in Entpd1+/− animals. Data are presented as means ± SEM (n = 4–6 per group). ***p < 0.001 (Student’s t-test) vs. wild-type aorta homogenate. (PDF 212 kb)
Fig. S3
Orbital shear stress induces upregulation of CD39 mRNA expression in vitro. Endothelial cells (three independent wells) were exposed to orbital shear stress (210 rpm = 11.5 dynes/cm2) for 24 h by using an orbital shaker as previously described (Dardik et al. J. Vasc. Surg 2005). CD39 mRNA expression was normalized to the reference gene and compared to cells cultured in static condition. Data represent the mean ± SEM. **p < 0.01 (Student’s t-test) vs. control cells. (PDF 37.1 kb)
Table S1
Source and concentration of molecules used to stimulate vascular cells (PDF 173 kb)
Table S2
Sequences of primer pairs used for RT-qPCR (PDF 118 kb)
Table S3
Gene expression variation evaluated by RT-qPCR in ECs and VSMCs submitted to cyclic stretch (15%, 0.5 Hz). Data are presented as means ± SEM of five independent VSMC cultures and three (for 72 h) or five (for 6, 24 h) independent EC cultures. * p < 0.05 (Student’s t-test) vs. time-matched control. (PDF 116 kb)
ESM 1
(DOCX 28.2 kb)
Rights and permissions
About this article
Cite this article
Roy, C., Tabiasco, J., Caillon, A. et al. Loss of vascular expression of nucleoside triphosphate diphosphohydrolase-1/CD39 in hypertension. Purinergic Signalling 14, 73–82 (2018). https://doi.org/10.1007/s11302-017-9597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-017-9597-9