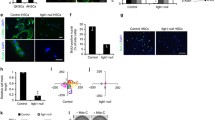
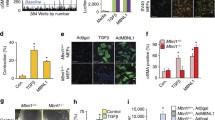
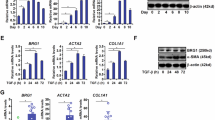
Abstract
Hepatic fibrosis represents a pathological wound healing and tissue repair process triggered in response to chronic liver injury. A heterogeneous population of activated non-parenchymal liver cells, known as liver myofibroblasts, functions as the effector cells in hepatic fibrosis. Upon activation, liver myofibroblasts become fibrogenic, acquiring contractile properties and increasing collagen production capacity, while developing enhanced sensitivity to endogenous molecules and factors released in the local microenvironment. Hepatic extracellular adenosine is a bioactive small molecule, increasingly recognized as an important regulator of liver myofibroblast functions, and an important mediator in the pathogenesis of liver fibrosis overall. Remarkably, ecto-5′-nucleotidase/Nt5e/Cd73 enzyme, which accounts for the dominant adenosine-generating activity in the extracellular medium, is expressed by activated liver myofibroblasts. However, the molecular signals regulating Nt5e gene expression in liver myofibroblasts remain poorly understood. Here, we show that activated mouse liver myofibroblasts express Nt5e gene products and characterize the putative Nt5e minimal promoter in the mouse species. We describe the existence of an enhancer sequence upstream of the mouse Nt5e minimal promoter and establish that the mouse Nt5e minimal promoter transcriptional activity is negatively regulated by an Elf2-like Ets-related transcription factor in activated mouse liver myofibroblasts.







Similar content being viewed by others
References
Lee YA, Wallace MC, Friedman SL (2015) Pathobiology of liver fibrosis: a translational success story. Gut 64:830–841
Hernandez-Gea V, Friedman SL (2011) Pathogenesis of liver fibrosis. Annu Rev Pathol 6:425–456
Novo E, Cannito S, Morello E, Paternostro C, Bocca C, Miglietta A, Parola M (2015) Hepatic myofibroblasts and fibrogenic progression of chronic liver diseases. Histol Histopathol 30:1011–1032
Fausther M, Lavoie EG, Dranoff JA (2013) Contribution of myofibroblasts of different origins to liver fibrosis. Curr Pathobiol Rep 1:225–230
Xu J, Liu X, Koyama Y, Wang P, Lan T, Kim IG, Kim IH, Ma HY, Kisseleva T (2014) The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front Pharmacol 5:167
Ferrari D, Gambari R, Idzko M, Muller T, Albanesi C, Pastore S, La Manna G, Robson SC, and Cronstein B (2015) Purinergic signaling in scarring. FASEB J
Wang H, Guan W, Yang W, Wang Q, Zhao H, Yang F, Lv X, Li J (2014) Caffeine inhibits the activation of hepatic stellate cells induced by acetaldehyde via adenosine A2A receptor mediated by the cAMP/PKA/SRC/ERK1/2/P38 MAPK signal pathway. PLoS One 9:e92482
Che J, Chan ES, Cronstein BN (2007) Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol Pharmacol 72:1626–1636
Yang P, Han Z, Chen P, Zhu L, Wang S, Hua Z, Zhang J (2010) A contradictory role of A1 adenosine receptor in carbon tetrachloride- and bile duct ligation-induced liver fibrosis in mice. J Pharmacol Exp Ther 332:747–754
Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN (2006) Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol 148:1144–1155
Hsu SJ, Lee FY, Wang SS, Hsin IF, Lin TY, Huang HC, Chang CC, Chuang CL, Ho HL, Lin HC, Lee SD (2015) Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology 61:1672–1684
Vecchio EA, White PJ and May LT (2017) Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front Pharmacol 8:243. doi:10.3389/fphar.2017.00243
Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2:351–360
Fausther M, Lecka J, Soliman E, Kauffenstein G, Pelletier J, Sheung N, Dranoff JA, Sevigny J (2012) Coexpression of ecto-5′-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver. Am J Physiol Gastrointest Liver Physiol 302:G447–G459
Fausther M, Sheung N, Saiman Y, Bansal MB, Dranoff JA (2012) Activated hepatic stellate cells upregulate transcription of ecto-5′-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am J Physiol Gastrointest Liver Physiol 303:G904–G914
Ji J, Yu F, Ji Q, Li Z, Wang K, Zhang J, Lu J, Chen L, E Q, Zeng Y, Ji Y (2012) Comparative proteomic analysis of rat hepatic stellate cell activation: a comprehensive view and suppressed immune response. Hepatology (Baltimore, Md) 56:332–349
Berardis S, Lombard C, Evraerts J, El Taghdouini A, Rosseels V, Sancho-Bru P, Lozano JJ, van Grunsven L, Sokal E, Najimi M (2014) Gene expression profiling and secretome analysis differentiate adult-derived human liver stem/progenitor cells and human hepatic stellate cells. PLoS One 9:e86137
Koyama Y, Wang P, Liang S, Iwaisako K, Liu X, Xu J, Zhang M, Sun M, Cong M, Karin D, Taura K, Benner C, Heinz S, Bera T, Brenner DA, Kisseleva T (2017) Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J Clin Invest 127:1254–1270
Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP (2002) Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110:993–1002
Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, Robson SC, Eberl G, Pallandre JR, Borg C, Ryffel B, Apetoh L, Rebe C, Ghiringhelli F (2012) Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36:362–373
Regateiro FS, Cobbold SP, Waldmann H (2013) CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol 171:1–7
Peng Z, Fernandez P, Wilder T, Yee H, Chiriboga L, Chan ES, Cronstein BN (2008) Ecto-5′-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB J 22:2263–2272
Kruglov EA, Jain D, Dranoff JA (2002) Isolation of primary rat liver fibroblasts. J Investig Med 50:179–184
Jainchill JL, Aaronson SA, Todaro GJ (1969) Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol 4:549–553
Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, Tarocchi M, Abar OT, Huang H, Sninsky JJ, Friedman SL (2009) Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology 49:960–968
Meurer SK, Alsamman M, Sahin H, Wasmuth HE, Kisseleva T, Brenner DA, Trautwein C, Weiskirchen R, Scholten D (2013) Overexpression of endoglin modulates TGF-beta1-signalling pathways in a novel immortalized mouse hepatic stellate cell line. PLoS One 8:e56116
Weiskirchen R, Gressner AM (2005) Isolation and culture of hepatic stellate cells. Methods Mol Med 117:99–113
Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG (2004) Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 287:G417–G424
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Oettgen P, Akbarali Y, Boltax J, Best J, Kunsch C, Libermann TA (1996) Characterization of NERF, a novel transcription factor related to the Ets factor ELF-1. Mol Cell Biol 16:5091–5106
Chrisman HR, Tindall DJ (2003) Identification and characterization of a consensus DNA binding element for the zinc finger transcription factor TIEG/EGRalpha. DNA Cell Biol 22:187–199
Karsenty G, de Crombrugghe B (1991) Conservation of binding sites for regulatory factors in the coordinately expressed alpha 1 (I) and alpha 2 (I) collagen promoters. Biochem Biophys Res Commun 177:538–544
McPherson LA, Weigel RJ (1999) AP2alpha and AP2gamma: a comparison of binding site specificity and trans-activation of the estrogen receptor promoter and single site promoter constructs. Nucleic Acids Res 27:4040–4049
Andrade CM, Roesch GC, Wink MR, Guimaraes EL, Souza LF, Jardim FR, Guaragna RM, Bernard EA, Margis R, Borojevic R, Battastini AM, Guma FC (2008) Activity and expression of ecto-5′-nucleotidase/CD73 are increased during phenotype conversion of a hepatic stellate cell line. Life Sci 82:21–29
Sharrocks AD (2001) The ETS-domain transcription factor family. Nat Rev Mol Cell Biol 2:827–837
Oikawa T, Yamada T (2003) Molecular biology of the Ets family of transcription factors. Gene 303:11–34
Friedman SL (2008) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88:125–172
Hahne JC, Okuducu AF, Fuchs T, Florin A, Wernert N (2011) Identification of ETS-1 target genes in human fibroblasts. Int J Oncol 38:1645–1652
Ozaki I, Zhao G, Mizuta T, Ogawa Y, Hara T, Kajihara S, Hisatomi A, Sakai T, Yamamoto K (2002) Hepatocyte growth factor induces collagenase (matrix metalloproteinase-1) via the transcription factor Ets-1 in human hepatic stellate cell line. J Hepatol 36:169–178
Hahne JC, Fuchs T, El Mustapha H, Okuducu AF, Bories JC, Wernert N (2006) Expression pattern of matrix metalloproteinase and TIMP genes in fibroblasts derived from Ets-1 knock-out mice compared to wild-type mouse fibroblasts. Int J Mol Med 18:153–159
Shirasaki F, Makhluf HA, LeRoy C, Watson DK, Trojanowska M (1999) Ets transcription factors cooperate with Sp1 to activate the human tenascin-C promoter. Oncogene 18:7755–7764
Czuwara-Ladykowska J, Shirasaki F, Jackers P, Watson DK, Trojanowska M (2001) Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J Biol Chem 276:20839–20848
Leask A, Chen S, Pala D, Brigstock DR (2008) Regulation of CCN2 mRNA expression and promoter activity in activated hepatic stellate cells. J Cell Commun Signal 2:49–56
Hollenhorst PC, Shah AA, Hopkins C, Graves BJ (2007) Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev 21:1882–1894
Selvaraj N, Kedage V, Hollenhorst PC (2015) Comparison of MAPK specificity across the ETS transcription factor family identifies a high-affinity ERK interaction required for ERG function in prostate cells. Cell Commun Signal 13:12
Tootle TL, Rebay I (2005) Post-translational modifications influence transcription factor activity: a view from the ETS superfamily. BioEssays 27:285–298
Baran CP, Fischer SN, Nuovo GJ, Kabbout MN, Hitchcock CL, Bringardner BD, McMaken S, Newland CA, Cantemir-Stone CZ, Phillips GS, Ostrowski MC, Marsh CB (2011) Transcription factor ets-2 plays an important role in the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol 45:999–1006
Snider NT, Griggs NW, Singla A, Moons DS, Weerasinghe SV, Lok AS, Ruan C, Burant CF, Conjeevaram HS, Omary MB (2013) CD73 (ecto-5′-nucleotidase) hepatocyte levels differ across mouse strains and contribute to mallory-denk body formation. Hepatology 58:1790–1800
Cheng J, Fei M, Fei M, Sang X, Sang X, Cheng Z, Gui S, Zhao X, Sheng L, Sun Q, Hu R, Wang L, Hong F (2014) Gene expression profile in chronic mouse liver injury caused by long-term exposure to CeCl3. Environ Toxicol 29:837–846
Peng Z, Borea PA, Varani K, Wilder T, Yee H, Chiriboga L, Blackburn MR, Azzena G, Resta G, Cronstein BN (2009) Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest 119:582–594
Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK (2011) Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol 186:4367–4374
Kristensen DB, Kawada N, Imamura K, Miyamoto Y, Tateno C, Seki S, Kuroki T, Yoshizato K (2000) Proteome analysis of rat hepatic stellate cells. Hepatology (Baltimore, Md) 32:268–277
Schmid TC, Loffing J, Le Hir M, Kaissling B (1994) Distribution of ecto-5′-nucleotidase in the rat liver: effect of anaemia. Histochemistry 101:439–447
Acknowledgements
Funding was provided by Gilead Sciences Research Scholars Program in Liver Disease Award UAMS# 271G141616-01 to MF, NIH – NIDDK R56DK076735 to JAD, and NIH – NCRR/NIH –NCATS #UL1TR000039 - University of Arkansas for Medical Sciences Institutional Support.
Author information
Authors and Affiliations
Contributions
MF designed, performed, and analyzed the experiments shown in Figs. 1, 2, 3, 4, 5, 6, conceived the study, and wrote the paper. EGL designed, performed, and analyzed the experiments shown in Figs. 1 and 6. JRG performed and provided technical assistance for experiments shown in Figs. 1 and 6. JAD conceived the study and edited the paper. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Michel Fausther declares that he has no conflict of interest.
Elise G. Lavoie declares that she has no conflict of interest.
Jessica R. Goree declares that she has no conflict of interest.
Jonathan A. Dranoff declares that he has no conflict of interest.
Ethical approval
All mouse experiments were performed in accordance with regulations approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Fausther, M., Lavoie, E.G., Goree, J.R. et al. An Elf2-like transcription factor acts as repressor of the mouse ecto-5′-nucleotidase gene expression in hepatic myofibroblasts. Purinergic Signalling 13, 417–428 (2017). https://doi.org/10.1007/s11302-017-9570-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-017-9570-7