Abstract
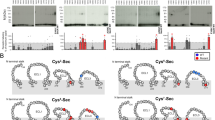
The P2X7 receptor (P2X7R) is a member of the ATP-gated ion channel family that exhibits distinct electrophysiological and pharmacological properties. This includes low sensitivity to ATP, lack of desensitization, a sustained current growth during prolonged receptor stimulation accompanied with development of permeability to large organic cations, and the coupling of receptor activation to cell blebbing and death. The uniquely long C-terminus of P2X7R accounts for many of these receptor-specific functions. The aim of this study was to understand the role of conserved ectodomain cysteine residues in P2X7R function. Single- and double-point threonine mutants of C119–C168, C129–C152, C135–C162, C216–C226, and C260–C269 cysteine pairs were expressed in HEK293 cells and studied using whole-cell current recording. All mutants other than C119T-P2X7R responded to initial and subsequent application of 300-μM BzATP and ATP with small amplitude monophasic currents or were practically nonfunctional. The mutagenesis-induced loss of function was due to decreased cell-surface receptor expression, as revealed by assessing levels of biotinylated mutants. Coexpression of all double mutants with the wild-type receptor had a transient or, in the case of C119T/C168T double mutant, sustained inhibitory effect on receptor trafficking. The C119T-P2X7R mutant was expressed on the plasma membrane and was fully functional with a slight decrease in the sensitivity for BzATP, indicating that interaction of liberated Cys168 with another residue rescues the trafficking of receptor. Thus, in contrast to other P2XRs, all disulfide bonds of P2X7R are individually essential for the proper receptor trafficking.





Similar content being viewed by others
References
Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandona D, Markwardt F, Schmalzing G, Di Virgilio F (2010) Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J 24:3393–404
Becker D, Woltersdorf R, Boldt W, Schmitz S, Braam U, Schmalzing G, Markwardt F (2008) The P2X7 carboxyl tail is a regulatory module of P2X7 receptor channel activity. J Biol Chem 283:25725–34
Bobanovic LK, Royle SJ, Murrell-Lagnado RD (2002) P2X receptor trafficking in neurons is subunit specific. J Neurosci 22:4814–24
Boumechache M, Masin M, Edwardson JM, Gorecki DC, Murrell-Lagnado R (2009) Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem 284:13446–54
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Clyne JD, Wang LF, Hume RI (2002) Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J Neurosci 22:3873–80
Cockcroft S, Gomperts BD (1979) ATP induces nucleotide permeability in rat mast cells. Nature 279:541–2
Coddou C, Acuna-Castillo C, Bull P, Huidobro-Toro JP (2007) Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of CYS132 for zinc potentiation and ASP138 for copper inhibition. J Biol Chem 282:36879–86
Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS (2011) Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63:641–83
Denlinger LC, Sommer JA, Parker K, Gudipaty L, Fisette PL, Watters JW, Proctor RA, Dubyak GR, Bertics PJ (2003) Mutation of a dibasic amino acid motif within the C terminus of the P2X7 nucleotide receptor results in trafficking defects and impaired function. J Immunol 171:1304–11
Di Virgilio F, Ferrari D, Adinolfi E (2009) P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal 5:251–6
Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR (1998) Cytolytic P2X purinoceptors. Cell Death Differ 5:191–9
Dubyak GR (2000) Purinergic signaling at immunological synapses. J Auton Nerv Syst 81:64–8
Egan TM, Cox JA, Voigt MM (2004) Molecular structure of P2X receptors. Curr Top Med Chem 4:821–9
Ennion SJ, Evans RJ (2002) Conserved cysteine residues in the extracellular loop of the human P2X(1) receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol Pharmacol 61:303–11
Feng YH, Li X, Wang L, Zhou L, Gorodeski GI (2006) A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem 281:17228–37
Friday SC, Hume RI (2008) Contribution of extracellular negatively charged residues to ATP action and zinc modulation of rat P2X2 receptors. J Neurochem 105:1264–75
Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS (2009) Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: this dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol 297:C430–9
Gu BJ, Saunders BM, Petrou S, Wiley JS (2011) P2X(7) is a scavenger receptor for apoptotic cells in the absence of its ligand, extracellular ATP. J Immunol 187:2365–75
Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS (2000) Expression of P2X(7) purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X(7) receptors. Am J Physiol Cell Physiol 279:C1189–97
Gudipaty L, Humphreys BD, Buell G, Dubyak GR (2001) Regulation of P2X(7) nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol 280:C943–53
Hibell AD, Kidd EJ, Chessell IP, Humphrey PP, Michel AD (2000) Apparent species differences in the kinetic properties of P2X(7) receptors. Br J Pharmacol 130:167–73
Hu B, Senkler C, Yang A, Soto F, Liang BT (2002) P2X4 receptor is a glycosylated cardiac receptor mediating a positive inotropic response to ATP. J Biol Chem 277:15752–7
Chaumont S, Jiang LH, Penna A, North RA, Rassendren F (2004) Identification of a trafficking motif involved in the stabilization and polarization of P2X receptors. J Biol Chem 279:29628–38
Jones CA, Vial C, Sellers LA, Humphrey PP, Evans RJ, Chessell IP (2004) Functional regulation of P2X6 receptors by N-linked glycosylation: identification of a novel alpha beta-methylene ATP-sensitive phenotype. Mol Pharmacol 65:979–85
Kawate T, Michel JC, Birdsong WT, Gouaux E (2009) Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460:592–8
Khakh BS, North RA (2006) P2X receptors as cell-surface ATP sensors in health and disease. Nature 442:527–32
Kim M, Spelta V, Sim J, North RA, Surprenant A (2001) Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem 276:23262–7
Lenertz LY, Wang Z, Guadarrama A, Hill LM, Gavala ML, Bertics PJ (2010) Mutation of putative N-linked glycosylation sites on the human nucleotide receptor P2X7 reveals a key residue important for receptor function. Biochemistry 49:4611–9
Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377:432–5
Li X, Zhou L, Feng YH, Abdul-Karim FW, Gorodeski GI (2006) The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol Biomarkers Prev 15:1906–13
Mackenzie AB, Young MT, Adinolfi E, Surprenant A (2005) Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem 280:33968–76
Marquez-Klaka B, Rettinger J, Bhargava Y, Eisele T, Nicke A (2007) Identification of an intersubunit cross-link between substituted cysteine residues located in the putative ATP binding site of the P2X1 receptor. J Neurosci 27:1456–66
Newbolt A, Stoop R, Virginio C, Surprenant A, North RA, Buell G, Rassendren F (1998) Membrane topology of an ATP-gated ion channel (P2X receptor). J Biol Chem 273:15177–82
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–67
Raouf R, Chakfe Y, Blais D, Speelman A, Boue-Grabot E, Henderson D, Seguela P (2004) Selective knock-down of P2X7 ATP receptor function by dominant-negative subunits. Mol Pharmacol 65:646–54
Rettinger J, Aschrafi A, Schmalzing G (2000) Roles of individual N-glycans for ATP potency and expression of the rat P2X1 receptor. J Biol Chem 275:33542–7
Roger S, Pelegrin P, Surprenant A (2008) Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci 28:6393–401
Rokic M, Tvrdonova V, Vavra V, Jindrichova M, Obsil T, Stojilkovic SS, Zemkova H (2010) Roles of Conserved Ectodomain Cysteines of the Rat P2X4 Purinoreceptor in Agonist Binding and Channel Gating. Physiol Res 59:927–935
Royle SJ, Bobanovic LK, Murrell-Lagnado RD (2002) Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem 277:35378–85
Smart ML, Gu B, Panchal RG, Wiley J, Cromer B, Williams DA, Petrou S (2003) P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem 278:8853–60
Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, Di Virgilio F, Monzani F (2008) Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology 149:389–96
Steinberg TH, Newman AS, Swanson JA, Silverstein SC (1987) ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem 262:8884–8
Surprenant A, North RA (2009) Signaling at purinergic P2X receptors. Annu Rev Physiol 71:333–59
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–8
Torres GE, Egan TM, Voigt MM (1998) N-linked glycosylation is essential for the functional expression of the recombinant P2X2 receptor. Biochemistry 37:14845–51
Vacca F, Giustizieri M, Ciotti MT, Mercuri NB, Volonte C (2009) Rapid constitutive and ligand-activated endocytic trafficking of P2X receptor. J Neurochem 109:1031–41
Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A (1999) Pore dilation of neuronal P2X receptor channels. Nat Neurosci 2:315–21
Visentin S, Renzi M, Frank C, Greco A, Levi G (1999) Two different ionotropic receptors are activated by ATP in rat microglia. J Physiol 519(Pt 3):723–36
Yan Z, Khadra A, Li S, Tomic M, Sherman A, Stojilkovic SS (2010) Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 30:14213–24
Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS (2008) The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol 132:563–73
Yi CL, Liu YW, Xiong KM, Stewart RR, Peoples RW, Tian X, Zhou L, Ai YX, Li ZW, Wang QW, Li CY (2009) Conserved extracellular cysteines differentially regulate the inhibitory effect of ethanol in rat P2X4 receptors. Biochem Biophys Res Commun 381:102–6
Acknowledgment
This study was supported by the Internal Grant Agency of Academy of Sciences (Grant No. IAA500110910), the Grant Agency of the Czech Republic (305/07/0681, 305/08/H037 and P304/12/P371), the Academy of Sciences of the Czech Republic (Research Project No. AVOZ 50110509), the Centrum for Neuroscience (Research Project No. LC554), and the Intramural Research Program of the NICHD, NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jindrichova, M., Kuzyk, P., Li, S. et al. Conserved ectodomain cysteines are essential for rat P2X7 receptor trafficking. Purinergic Signalling 8, 317–325 (2012). https://doi.org/10.1007/s11302-012-9291-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-012-9291-x